Table of Contents
Air pollution
Introduction
- Air pollution refers to the change in the composition of the atmosphere by gases or other material released from Earth’s surface.
- Pollutants: natural e.g. volcanic ash or anthropogenic e.g. smoke, gases [compared to human activities, contribution of volcanoes in air pollution is minuscule]
- Human reasons: started primarily since discovery of fire
- Air pollution increased markedly during the Industrial Revolution and accelerated during the latter half of the twentieth century.
Natural pollutants
- Volcanoes: sulfur, VOC, acid mist, H2S,
- Sea spray and decaying vegetation: sulfur
- Bacterial metabolism of vegetation: responsible for 2/3 methane in env
Classification of air pollutants
- Air pollutants are classified as being primary or secondary
- compounds.
- Primary pollutants are directly given off by a particular process or activity.
- Examples: Emissions from a factory smokestack, the tailpipe of an idling car, or the ash propelled out of an erupting volcano
- Secondary pollutants are not given off directly, but rather form in the air when other compounds interact with one another.
- Examples: are particles that form when gases in the air interact with another pollutant that is a mixture of smoke and SO2 (better known as smog), peroxyacetyl nitrate (which is formed by the interaction of nitrogen oxides and volatile organic compounds), and ground-level ozone that forms near Earth’s surface.
Criteria pollutants
- US clean air act 1970
- 6 major ‘criteria pollutants’ (largest quantity of air pollution and most serious threat posers)
- sulfur dioxide,
- Nitrogen oxides,
- carbon monoxide,
- ozone,
- lead, and
- particulate matter
- National ambient air quality standards (NAAQS) devised for them
- Other air pollutants include heavy metals and both chemical and biological toxins.
- Some air pollutants are able to persist in the atmosphere for a long time. Known as persistent organic pollutants, compounds such as dioxin and furan may be transported long distances and so have the potential to affect people far from their point of release.
- Fugitive emissions are those that do not go through a smokestack. e.g. dust from soil erosion, mining, rock crushing, leaks etc.
Carbon Monoxide (CO)
- Carbon monoxide (CO) is a colorless, odorless, nonirritating, but highly toxic gas.
- produced mainly by incomplete combustion of fuel (coal, oil, charcoal, or gas), as in furnaces, incinerators, engines, or fires, as well as in decomposition of organic matter. Land-clearing fires and cooking fires also are major sources
- CO blocks oxygen uptake in blood by binding irreversibly to hemoglobin
- Human activities produce 50 pc of CO released to the atmosphere each year.
- About 90 percent of the CO in the air is converted to CO 2 in photochemical reactions that produce ozone.
Nitrogen Oxides (NO2 )
- Nitrogen oxides are highly reactive gases formed when nitrogen in fuel or in air is heated (during combustion) to temperatures above 650°C (1,200°F) in the presence of oxygen.
- Bacteria can also form NO as they oxidize nitrogen-containing compounds in soil or water
- Nitrogen oxide (NO2 ), a reddish-brown gas that gives photochemical smog its distinctive color.
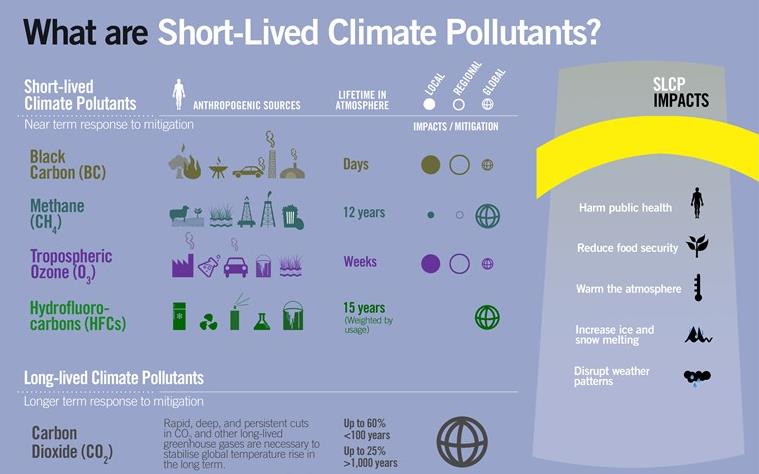
- Nitrous oxide absorbs ultraviolet light and is an important greenhouse gas.
- Human activities: 60 pc of global emissions
- Sources: Fuel combustion and electric power generation, fertilizers
Sulfur Dioxide (SO2)
- Natural sources: evaporation of sea spray, erosion of sulfate- containing dust from arid soils, fumes from volcanoes and hot springs, and biogenic emissions of hydrogen sulfide (H2S) and organic sulfur- containing compounds.
- Anthropogenic sources responsible for 90 pc of sulfur in urban air
- China and US are largest polluters of S: coal burning and smelting
Ozone (O3 ) and Photochemical Oxidants
- Ozone (O3 ) high in the stratosphere provides a valuable shield for the biosphere by absorbing incoming ultraviolet radiation.
- But at ground level, O3 is a strong oxidizing reagent that damages vegetation, building materials (such as paint, rubber, and plastics), and sensitive tissues (such as eyes and lungs).
- acrid, biting odor that is a distinctive characteristic of photochemical smog.
- Secondary pollutant: product of photochemical reactions between other pollutants, such as NOx or VOC
- Plants are the largest source of VOCs
Lead
- Toxic to nervous system
- Industrial and mining processes produce it
- Since ban on leaded fuel in US, avg IQ has risen by 3 points
- Many countries have renounced leaded gasoline now
Particulate Matter
- solid particles or liquid droplets suspended in a gaseous medium
- Aerosols: dust, ash, soot, lint, smoke, pollen, spores, algal cells
- reduce visibility and leave dirty deposits on windows, painted surfaces, and textiles
- PM 2.5 (fine particles): produced by burning power plants, exhausts,
- PM 10 (coarse particles): dust
Hazardous air pollutants (HAPs)
- include carcinogens, neurotoxins, mutagens, teratogens, endocrine system disrupters, and other highly toxic compounds
- bioaccumulative and toxic: mercury and dioxins
Other pollutants
- About 75 percent of human exposure to mercury comes from eating fish.
- Global air circulation also deposits airborne mercury on land.
- CO2
- Halogens
Atmospheric Processes
- Topography, climate, and physical processes in the atmosphere play an important role in the transport, concentration, dispersal, and removal of many air pollutants.
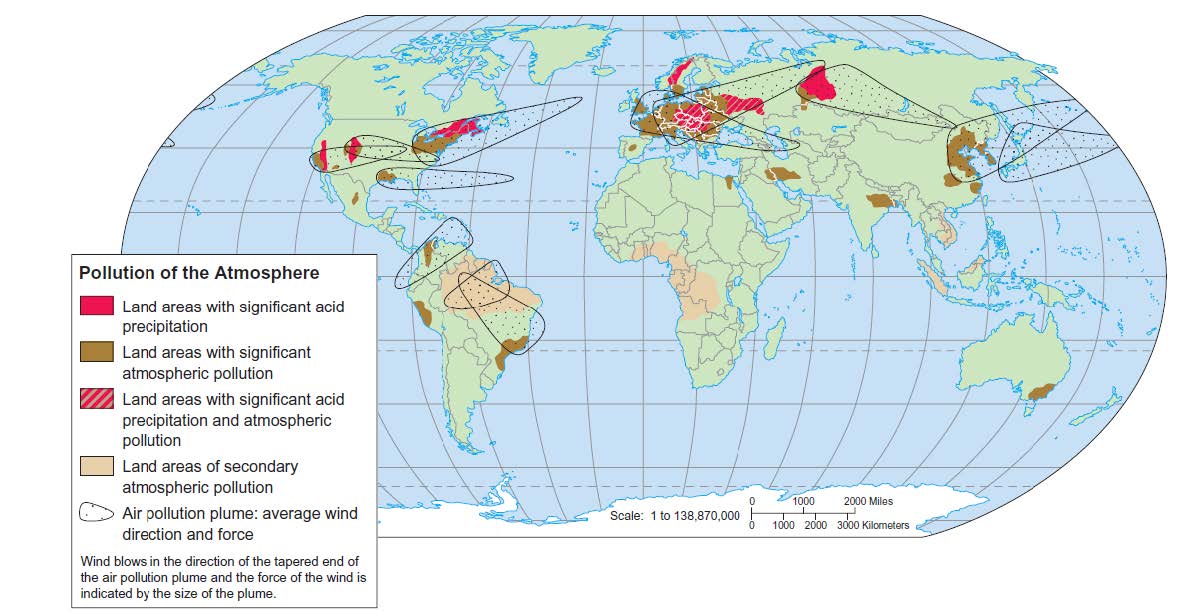
Wind currents carry pollutants worldwide
-
- Studies of air pollutants over southern Asia reveal a 3 km thick toxic cloud of ash, acids, aerosols, dust, and photo chemical reactants that regularly covers the entire Indian subcontinent and can last for much of the year.
- Around 2 million ppl in India die each year of air pollution
- 80 pc of this cloud is human made
- Meteorologists: may disrupt monsoon, rice harvest and rain
- A process called “grasshopper” transport, or atmosphere distillation, helps deliver contaminants to the poles.
- Bioaccumulation occurs: Whales, polar bears, sharks, and other top carnivores in polar regions have been shown to have dangerously high levels of pesticides, metals, and other HAPs in their bodies.
- The Inuit people of Broughton Island, well above the Arctic Circle, have higher levels of polychlorinated biphenyls (PCBs) in their blood than any other known population, except victims of industrial accidents (due to contaminated fish!)
- This exacerbates the cultural crisis caused by climate change.
Stratospheric ozone is destroyed by chlorine (CFCs)
- A 1 percent loss of ozone could result in about a million extra human skin cancers per year worldwide,
- Excessive UV exposure could reduce agricultural production and disrupt ecosystems
- About 10 percent of all stratospheric ozone worldwide has been destroyed in recent years
- Montreal protocol: Global CFC production has been cut by more than 95 percent since 1988
Impacts and Issues
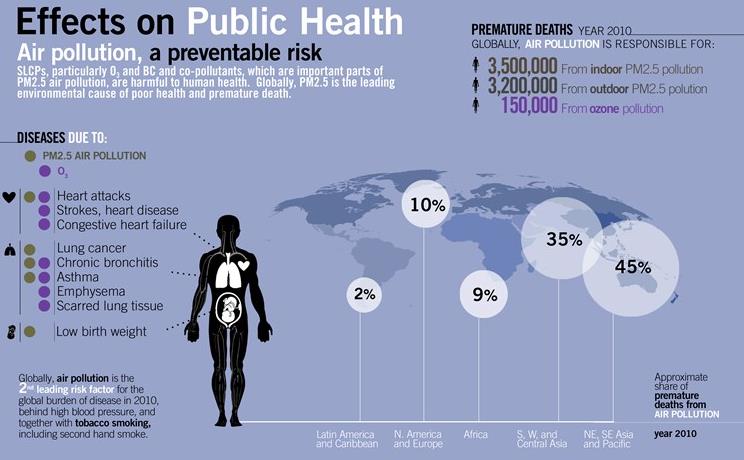
- These include pollution-aggravated bronchitis, asthma, emphysema, lung disease, heart disease, and allergic reactions.
- graphic example: December 1952- weeklong formation of smog over London, England, attributed to the immediate death of more than 4,000 people and the respiratory- and heart-related deaths over the next few months of 8,000 more.
- 6 billion lb. of toxins released in air each year [US EPA]
- China, Beijing: produced highest level of nitrogen oxide ever (respiratory irritant)
- Main reason of GW
Polluted air damages lungs
- Routes of pollution: inhalation, assimilation (digestion), absorption
- nearly 5 to 6 million people die each year from deaths that are the direct result of air pollution (WHO).
- Heart attacks, respiratory diseases, and lung cancer
- at least 1.3 billion people around the world live in areas where outdoor air is dangerously polluted [UN]
- Mexico city – one of the most polluted cities of the world
Plants suffer cell damage and lost productivity
Acid deposition has many negative effects
-
- acid precipitation (the deposition of wet acidic solutions or dry acidic particles from the air)
- Acid rain can disrupt reproduction in fish
- Results in rapid loss of forests
- Destroys buildings and monuments: Taj mahal, David etc.
Smog and haze reduce visibility
Air Pollution Control
- Early approach: “Dilution is the solution to pollution”
- Made the problem worse
- Today solutions focus on transportation and energy sectors
Substances can be captured after combustion
- Particulate removal involves filtering air emissions e.g. electrostatic Precipitators, scrubbers etc.
- Pre-combustion cleaning
Fuel switching and fuel cleaning cut emissions
-
- Use low-sulfur coal
- Avoided leaded gasoline
- Switch to nuclear energy
- Renewable energy
Global Prospects
Rapid industrialization and urban growth outpace pollution controls
-
- Many of China’s 400,000 factories have no air pollution controls.
- Sixteen of the 20 cities in the world with the worst air quality are in China.