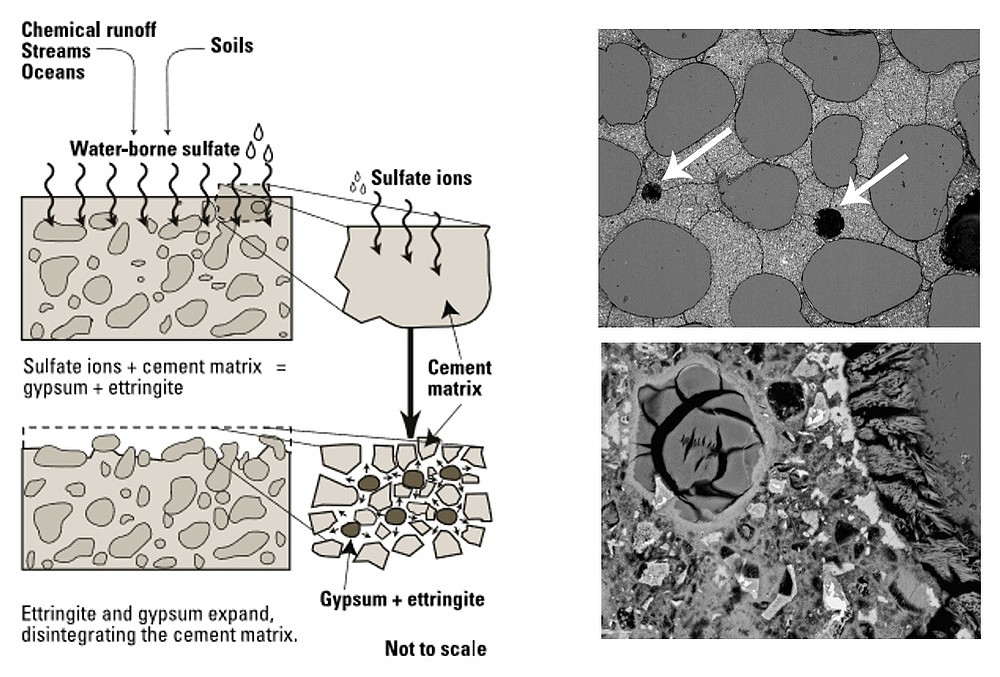
Sulphate attack is one of the most common phenomena that strikes hardened reinforced concrete to an extent that leads to its disintegration and splintering. For concrete structures, the environmental conditions must be thoroughly investigated and any scant indication likely to affect the structure’s strength or durability needs to be taken into account. Sulphate attack on concrete is nothing less than an appreciating muddle, the origin of which can be internal or external.
Internal sulphate attack in concrete involves a chemical reaction between celite (C3A or tricalcium aluminate) present in the cement paste and the sulphates present in the concrete mix. On the other hand, the external attack is commenced once the sulphates present in the soil or water react with calcium hydroxide.
Both these attacks prove deadly for concrete and therefore, appropriate measures are ought to be taken timely to prevent concrete from the surging occurrences of sulphate attacks. An overview on sulphate attack is given in the adjoining figure.
Fig.: An overview on sulphate attack on concrete
Table of Contents
Internal Sulphate Attack (ISA) in Concrete
At the onset of the hydration process, calcium sulphate (or gypsum) reacts with celite and forms ettringite or Aft (Alumino-ferrite-tri). During the early stages, ettringite formation in the voids, pores, and at the interface of aggregates and cement paste, proves advantageous for strength gain in concrete.
However, if gypsum is present in profusion, it remains in the hardened concrete and dissolves sulphate ions (SO42-). These ions further react with celite and form Aft, which proves cancerous for the concrete in terms of the volume expansion it causes. This is because in the later stages, the reaction products deplete the accommodation capacity of the previously intact internal structure.
Coupled with this augmented volume is the strength loss due to huge stresses generated in the hardened paste. As a consequence, the ability of the hardened concrete to bear the applied loading reduces substantially, and the structure is prone to collapse anytime soon.
C3A + 3CaSO4 → C3A.3CaSO4.32H2O (Ettringite)
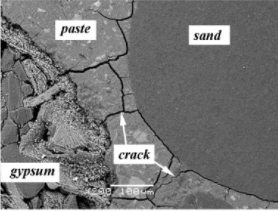
Fig.: SEM image of cracking pattern in concrete subjected to ISA
It is important to highlight that as the temperature hikes 70°C, the ettringite gets destroyed and this destruction propels the expansion process of concrete in the form of a network of cracks within the structure. The stresses produced are much higher than the tensile strength of the concrete and therefore, the cracks sprawl throughout the structure. The figure below shows the propagation of cracks due to an internal sulphate attack in a bridge structure.
Fig.: Internal sulphate attack in concrete
External Sulphate Attack (ESA) on Concrete
Sulphates (expressed as SO3) present in the ground (or sea) water or soil attack hardened, relatively permeable concrete and react chemically with calcium hydroxide. The sources of sulphates can be one of the following:
- Sodium sulphate
- Potassium sulphate
- Magnesium sulphate
The reaction product is calcium sulphate (CaSO4), also known as gypsum. Rationally, calcium hydroxide works in opposition to the strength-imparting compounds, and therefore, its presence is somewhat useless for the concrete. The product, calcium sulphate further reacts with the Bogue’s compound, tricalcium aluminate (C3A) and forms Alumino-ferrite-tri (Aft) or ettringite.
Unfortunately, this reaction proves to be a quagmire for the concrete structure at later stages. This is because the sequent reaction product results in a humungous increase in the volume of concrete. Quantifying the volume expansion, an increase of more than 200% will not be even a wee exaggeration.
As a consequence, the process of formation of voids in the concrete is stimulated and the possibility of saving the concrete from the scourge of moisture ingress practically drops to zero. Moisture makes its way inside the concrete, attacking the reinforcement and subsequently making corrosion inevitable.
Once the steel corrodes, the bond between the reinforcement and concrete at the interface is weakened and over time, the concrete is prone to spalling and disintegration.
Sulphur + Ca(OH)2 → CaSO4
3CaSO4 +C3A→ C3A.3CaSO4.32H2O (Ettringite)
Fig.: SEM image of ettringite
Fig.: External sulphate attack on a reinforced concrete column
Properties of sulphate-attacked concrete
The sulphate-attacked concrete possesses the following physical properties;
- It has a whitish appearance, that points fingers to efflorescence due to the leaching of calcium hydroxide.
- It is prone to cracking and disintegration.
Fig.: Efflorescence due to sulphate attack
Locations where concrete is vulnerable to sulphate attack
Sulphate attack is generally seen in the following locations;
- Sea-side structures i.e., the structures constructed in the vicinity of a water body and in contact with water. Therefore, the possibility of sulphate attacks on such structures is very high.
- Areas where the freeze-thaw process is common i.e., the fluctuation in atmospheric temperature compels the moisture to penetrate inside the concrete structure, making sulphate attack eminent.
- Water-connected structures, which are in constant exposure to water e.g., the bridge piers that are constructed inside the water body.
- Elevated temperatures or steam curing can become a stimulus for the chemical reactions to occur.
Remedial Measures
Preventing concrete from sulphate attack is one of the ways of ensuring the structure’s durability. Therefore, in areas where the concrete is vulnerable to the attack by sulphates, either external or internal, succumbing to the environmental conditions is not promising.
Hence, it is imperative to look for alternatives that work to either reduce the sulphate attack or seal the concrete from the same. The following remedial measures taken timely can help affirm the structure’s resistance to the attack of sulphates.
Against Internal Sulphate Attack
- Concrete should not be poured in hot weather. This is to prevent the accumulation of heat.
- In order to prevent excessive heat of hydration, slag or fly ash can be used along with cement.
- Delayed ettringite formation (DEF), which is the primary cause of internal sulphate attack can be reduced by using sulphate-resistant cement containing a lower percentage of celite.
Against External Sulphate Attack
External sulphate attack is majorly prevented by careful selection and proportion of materials used in the mix design of concrete. Following remedial measures can prove beneficial against ESA:
Use of special cements:
- Use sulphate-resistant cement (ASTM Type-V cement) in areas prone to sulphate attack.
- Use high alumina cement (HAC)
- Use Portland slag cement (PSC) with the percentage of slag exceeding 50%.
- Use super-sulphated cement (SC), in case of very high sulphate concentrations.
- Reduce the amount of tri-calcium aluminate (C3A), the presence of which triggers the production of calcium sulpho-aluminate or ettringite.
- Use Portland pozzolana cement which reduces the percentage of calcium hydroxide through pozzolanic action.
- Use air-entrained concrete that expels the extra stresses once the volume expansion takes place.
- Reduce the permeability of hardened concrete.
- While performing the mix design procedure, a low water-to-cement ratio helps in improving resistance to the action of sulphates.
- Use of secondary cementitious materials in predefined proportions can lower the permeability of concrete. For instance, ground granulated blast furnace slag (GGBFS) is experimentally found effective in preventing concrete from sulphate attacks.