Cement is one of the most common construction materials possessing cohesive as well adhesive properties. Therefore, its use as a binder or glue for various purposes serving construction activities is quite common around the world.
The fractions of various ingredients are proportioned in such a way that the resulting cement contains the desired properties. These percentages vary depending upon the type of cement to be manufactured and the target characteristics.
However, under ordinary conditions and for general construction works, ordinary Portland cement (OPC) is used which is hydraulic in nature. This is suggestive of the fact that when the cement reacts with water, a chemical reaction, frequently spoken of in concrete technology as hydration, commences and the durable product formed as a result of the chemical upend is the major player for imparting strength.
It is wondrous that this product formed by reacting with water becomes a water-repellant and resists the effect of water after gaining strength.
Detailed below are the raw materials and the properties they impart to the cement powder.
Table of Contents
Raw Materials for Ordinary Portland Cement Manufacturing
The crude materials required for manufacturing cement are either calcareous or argillaceous. Calcareous materials contain high content of lime whereas argillaceous ones comprise high percentages of silica and alumina. The former ones can be obtained from limestone, chalk, oyster shells, marl, etc. whereas the latter ones can be procured from clay, shale, slate, sand or selected blast furnace slag.
Following are the raw materials subjected to mixing and burning to form cement clinkers:
- Lime (CaO): Lime is obtained from calcareous rocks that might be present in the vicinity of a mountainous area.
- Silica (SiO2): The main source of silica is sand or some argillaceous rock.
- Alumina (Al2O3): This is a chief constituent of clay and is obtained from the same.
- Iron Oxide (Fe2O3): It is obtained from clay, fly ash or iron ore.
- Trace Ingredients: Magnesia (MgO) and sulphur trioxide (SO3) are present in trace amounts.
Percentage of Raw Materials Used
|
Oxide Ingredient |
Range (%) |
|
Lime (CaO) |
60-66 |
| Silica (SiO2) |
19-25 |
|
Alumina (Al2O3) |
3-8 |
|
Iron Oxide (Fe2O3) |
1-5 |
|
Magnesia (MgO) |
0-5 |
|
Sulphur Trioxide (SO3) |
1-3 |
Source: Portland Cement Association (PCA)
- The above ingredients are put in a kiln and burned at a very high temperature (approximately 1400 °C) to form cement clinkers. These clinkers are ground with a small quantity of gypsum to form powdered cement which is later packed and transported for the intended construction operations.
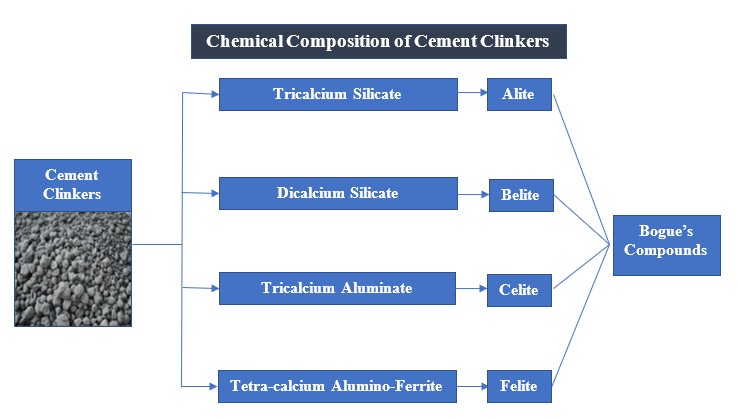
Fig.: Major raw materials for OPC manufacturing
Chemical Composition of Cement Clinkers
Cement clinkers contain 4 main compounds that are formed during the burning and mixing process of the crude ingredients. These compounds are called Bogue’s compounds and their details are given as follows:
Bogue’s Compounds:
During the burning process of raw materials for the manufacture of cement, a lot of chemical reactions take place and various compounds are formed as a result of the chemical whip. These compounds possess properties pertaining the setting and hardening in the presence of water. They were identified by Bogue and were named as ‘Bogue’s compounds.’ The chemical synthesis and properties of these compounds are given below;
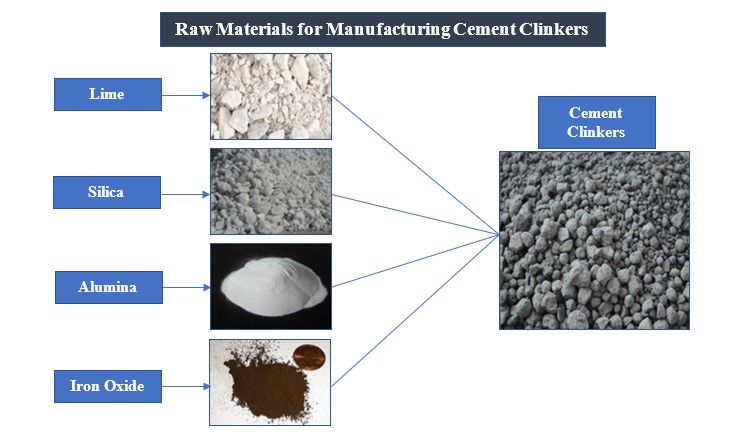
Fig.: Chemical composition of OPC Clinkers
1)Tricalcium Silicate (Alite)
- The chemical formula of tricalcium silicate is 3CaO.SiO2, which is abbreviated as C3S where C stands for calcium oxide, and S refers to silica.
- It contributes to a faster hydration process.
- It is responsible for early and overall strength gain in concrete.
- It protects the concrete from sulphate attacks.
Percentage in Ordinary Portland Cement:
The powdered cement contains about 50% tricalcium silicate.
2)Dicalcium Silicate (Belite)
- The chemical formula of this compound is 2CaO.SiO2 and is shortened as C2S (C for calcium oxide and S for silica).
- Belite is liable for gradual or late strength gain in concrete.
Percentage in Ordinary Portland Cement:
OPC contains almost 25% of this compound.
3)Tricalcium Aluminate (Celite)
- Celite has a chemical formula of 3CaO.Al2O3 and is compactly written as C3A where A is for alumina.
- It controls the initial setting time of concrete.
- A very large quantity of celite in the cement paste makes the concrete susceptible to the attack of sulphates.
Percentage in Ordinary Portland Cement:
About 10% of this compound is present in the final powdered cement.
4)Tetra-calcium Alumino-ferrite (Felite)
- This compound holds a chemical formula of 4CaO.Al2O3.Fe2O3, which is shortened as C4AF where F is for iron oxide.
- It is responsible for regulating the temperature of the mix, lowering the evolution of heat of hydration.
- It also has a scant contribution in the early strength gain process of concrete.
Percentage in Ordinary Portland Cement:
The powdered cement comprises about 10% of felite.
A small quantity of gypsum is added to the clinkers to further regulate the setting time of cement. However, the percentage is minuscule i.e., about 3-5%.





