Table of Contents
Introduction
Concrete is a complex material of construction that enables the high compressive strength of natural stone to be used in any configuration.
In tension, however, concrete can be no stronger than the bond between the cured cement and the surfaces of the aggregate. This is generally much lower than the compressive strength of the concrete. Concrete is therefore frequently reinforced, usually with steel.
When a system of steel bars or a steel mesh is incorporated in the concrete structure in such a way that the steel can support most of the tensile stresses and leave the immediately surrounding concrete comparatively free of tensile stress, then the complex is known as “reinforced concrete”.
Corrosion In Steel Reinforced Concrete
Reinforced Concrete
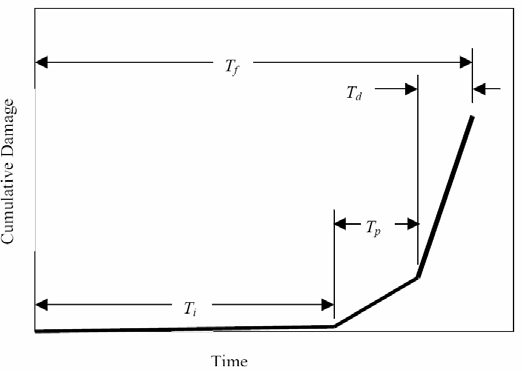
(2) Time, subsequent to corrosion initiation, for appearance of a crack on the external concrete surface (crack propagation),
(3) Time for surface cracks to progress into further damage and develop into spalls, to the point where the functional service life, Tf, is reached. Figure illustrates these schematically as a plot of cumulative damage versus time.
Common Corrosion Types
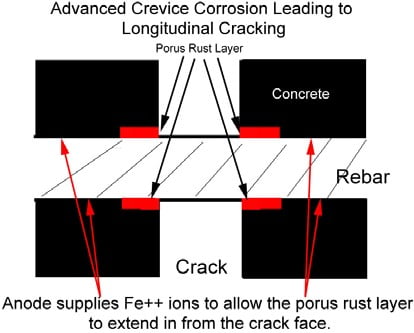
Crevice Corrosion
Crevice corrosion is a localized form of corrosion usually associated with a stagnant solution on the micro-environmental level. Such stagnant micro environments tend to occur in crevices (shielded areas). Oxygen in the liquid which is deep in the crevice is consumed by reaction with the metal.
Oxygen content of liquid at the mouth of the crevice which is exposed to the air is greater, so a local cell develops in which the anode, or area being attacked, is the surface in contact with the oxygen-depleted liquid.

 Pitting
Pitting
Theories of passivity fall into two general categories, one based on adsorption and the other on presence of a thin oxide film. Pitting in the former case arises as detrimental or activator species, such as Cl–, compete with O2 or OH– at specific surface sites.
By the oxide film theory, detrimental species become incorporated into the passive film, leading to its local dissolution or to development of conductive paths. Once initiated, pits propagate auto-catalytically according to the generalized reaction,
M+n + nH2O + nCl– → M(OH)n + nHCl, resulting in acidification of the active region and corrosion at an accelerated rate (M+n and M are the ionic and metallic forms of the corroding metal).
 Reasons Of Corrosion
Reasons Of Corrosion
The two most common causes of reinforcement corrosion are (i) localized breakdown of the passive film on the steel by chloride ions and (ii) general breakdown of passivity by neutralization of the concrete, predominantly by reaction with atmospheric carbon dioxide.
Sound concrete is an ideal environment for steel but the increased use of deicing salts and the increased concentration of carbon dioxide in modern environments principally due to industrial pollution, has resulted in corrosion of the rebar becoming the primary cause of failure of this material.
The scale of this problem has reached alarming proportions in various parts of the world. Following are the contributing factors leading to corrosion :
Loss of Alkalinity due to Carbonation
It is well known that if bright steel is left unprotected in the atmosphere a brown oxide rust quickly forms and will continue to grow until a scale flakes from the surface. This corrosion process will continue unless some external means is provided to prevent it.
One method is to surround the steel with an alkaline environment having a pH value within the range 9.5 to 13. At this pH value a passive film forms on the steel that reduces the rate of corrosion to a very low and harmless value. Thus, concrete cover provides chemical as well as physical protection to the steel. However, alkalinity can be lost as a result of:
- Reaction with acidic gases (such as carbon dioxide) in the atmosphere.
- Leaching by water from the surface.
Concrete is permeable and allows the slow ingress of the atmosphere; the acidic gases react with the alkalis (usually calcium, sodium and potassium hydroxides), neutralising them by forming carbonates and sulphates, and at the same time reducing the pH value.
If the carbonated front penetrates sufficiently deeply into the concrete to intersect with the concrete reinforcement interface, protection is lost and, since both oxygen and moisture are available, the steel is likely to corrode. The extent of the advance of the carbonation front depends, to a considerable extent, on the porosity and permeability of the concrete and on the conditions of the exposure.
In the case of carbonation, atmospheric carbon dioxide (CO2) reacts with pore water alkali according to the generalized reaction,
Ca(OH)2 + CO2 CaCO3 + H2O
It consumes alkalinity and reduces pore water pH to the 8–9 range, where steel is no longer passive.
 Loss of Alkanity due to Chlorides
Loss of Alkanity due to Chlorides
The passivity provided by the alkaline conditions can also be destroyed by the presence of chloride ions, even though a high level of alkalinity remains in the concrete. The chloride ion can locally de-passivate the metal and promote active metal dissolution.
Chlorides react with the calcium aluminate and calcium aluminoferrite in the concrete to form insoluble calcium chloroaluminates and calcium chloroferrites in which the chloride is bound in non-active form; however, the reaction is never complete and some active soluble chloride always remains in equilibrium in the aqueous phase in the concrete. It is this chloride in solution that is free to promote corrosion of the steel.
At low levels of chloride in the aqueous phase, the rate of corrosion is very small, but higher concentration increases the risks of corrosion.
Cracks due to Mechanical Loading

If the crack penetrates to the steel, protection can be lost. This is especially so under tensile loading, for debonding of steel and concrete occurs to some extent on each side of the crack, thus removing the alkaline environment and so destroying the protection in the vicinity of the debonding.
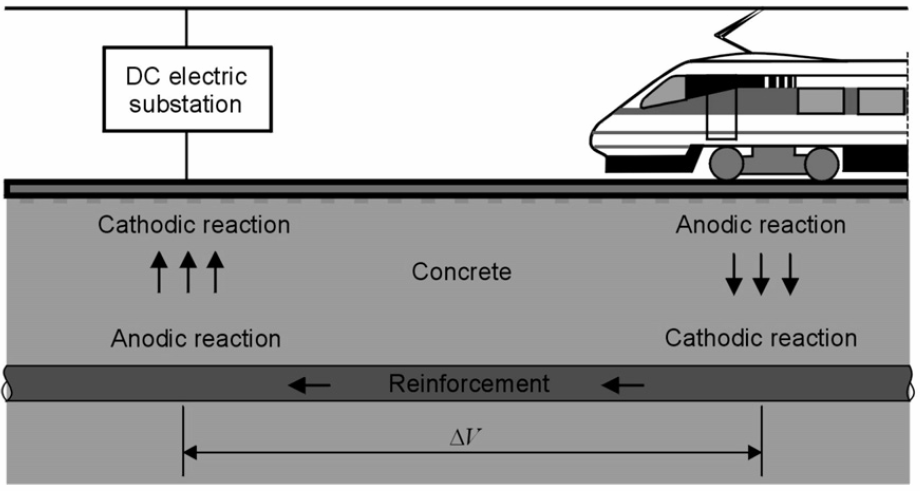
Stray Currents
Stray currents, arising for instance from railways, cathodic protection systems, or high voltage power lines, are known to induce corrosion on buried metal structures, leading to severe localized attack.
They may find a low resistance path by flowing through metallic structures buried in the soil (pipelines, tanks, industrial and marine structures). a cathodic reaction (e.g., oxygen reduction or hydrogen evolution) takes place where the current enters the buried structure, while an anodic reaction (e.g., metal dissolution) occurs where the current returns to the original path, through the soil.
Metal loss results at the anodic points, where the current leaves the structure; usually, the attack is extremely localised and can have dramatic consequences especially on pipelines.
Example of stray current from a DC railway line picked up by steel reinforcement in concrete.
Corrosion of steel reinforcement due to atmospheric pollution
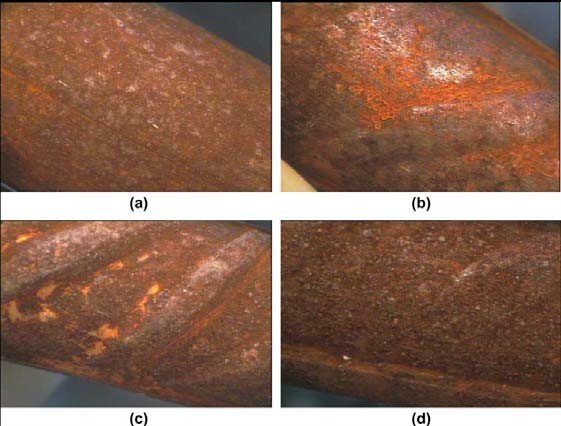
Most of the times steel reinforcement is exposed to the atmosphere during transportation and storage in the building sites for a long period before their installation in the concrete structures. At any of those stages, steel rebars can be contaminated by chloride ions from sea spray or windblown salt. This fact leads to the formation of corrosion products on their surface.
Fiber optical microscope images after three months at open atmosphere conditions.
Moisture Pathways
Overwatered leading to shrinkage cracking
If the surface of the concrete is subject to long-term wetting, the water will eventually reach the level of the reinforcement, either through diffusion through the porous structure of the concrete, or by traveling along cracks in the concrete.
Concrete roof decks, by their nature, are meant to be protected from moisture. However, the presence of moisture on roofing systems may result from failure of the roofing membrane, poor detailing of drainage facilities, or lack of maintenance of drainage facilities.
Water-Cement Ratio
Concrete placed with a high water-cement ratio, as seen under Freeze-thaw cycles, is more porous due to the presence of excess water in the plastic concrete. The porosity increases the rte of diffusion of water and electrolytes through the concrete and makes the concrete more susceptible to cracking.
Low Concrete Tensile Strength
Concrete with low tensile strength facilitates corrosion damage in two ways. First, the concrete develops tension or shrinkage cracks more easily, admitting moisture and oxygen, and in some cases chlorides, to the level of the reinforcement. Second, the concrete is more susceptible to developing cracks at the point that the reinforcement begins to corrode.
Electrical Contact with dissimilar metals
Dissimilar metals in contact initiate a flow of electrons that promotes the corrosion of one or the other, by a process known as galvanic corrosion. When two dissimilar metals are in contact with each other the more active metal (lower on the list) will induce corrosion on the less active. Such corrosion may induce cracking and damage in the concrete.
Corrosion due to difference in environments
Corrosion occurs when two different metals, or metals in different environments, are electrically connected in a moist or damp concrete.
This will occur when:
- Steel reinforcement is in contact with an aluminium conduit.
- Concrete pore water composition varies between adjacent or along reinforcing bars.
- Where there is a variation in alloy composition between or along reinforcing bars.
- Where there is a variation in residual/applied stress along or between reinforcing bars.
Prevention Methods
- Keep concrete always dry, so that there is no H2O to form rust. Also aggressive agents cannot easily diffuse into dry concrete. If concrete is always wet, then there is no oxygen to form rust.
- A polymeric coating is applied to the concrete member to keep out aggressive agents. A polymeric coating is applied to the reinforcing bars to protect them from moisture and aggressive agents. The embedded epoxy-coating on steel bars provide a certain degree of protection to the steel bars and, thereby, delay the initiation of corrosion. These coatings permit movement of moisture to the steel surface but restrict oxygen penetration such that a necessary reactant at cathodic sites is excluded.
- Stainless steel or cladded stainless steel is used in lieu of conventional black bars.
- FLY ASH : Using a Fly Ash concrete with very low permeability, which will delay the arrival of carbonation and chlorides at the level of the steel reinforcement. Fly Ash is a finely divided silica rich powder that, in itself, gives no benefit when added to a concrete mixture, unless it can react with the calcium hydroxide formed in the first few days of hydration. Together they form a calcium silica hydrate (CSH) compound that over time effectively reduces concrete diffusivity to oxygen, carbon dioxide, water and chloride ions.
- A portion of the chloride ions diffusing through the concrete can be sequestered in the concrete by combining them with the tricalcium aluminate to form a calcium chloro-aluminate (Friedel’s salt). It can have a significant effect in reducing the amount of available chlorides thereby reducing corrosion.
- Electrochemical injection of the organic base corrosion inhibitors, ethanolamine and guanidine, into carbonated concrete.
- The rougher the steel surface, the better it adheres to concrete. oxidation treatment (by water immersion and ozone exposure) of rebar increases the bond strength between steel and cement paste to a value higher than that attained by clean rebars. In addition, surface deformations on the rebar (such as ribs) enhance the bond due to mechanical interlocking between rebar and concrete.
- As the cement content of the concrete increases (for a fixed amount of chloride in the concrete), more chloride reacts to form solid phases, so reducing the amount in solution (and the risk of corrosion), and as the physical properties improve, the extent of carbonation declines, so preventing further liberation of chloride from the solid phase.
- Electrochemical Chloride Extraction (ECE) is a relatively new technology for which long-term service data are limited. This method employs a temporary anode that is operated at current density
orders of magnitude higher than for cathodic protection, such that anions, including chlorides, electromigrate away from the embedded steel cathode. Repassivation can then occur, similar to what was discussed above in conjunction with cathodic protection, although this occurs in a shorter period of time (1–2 weeks to several months). Not all chlorides are removed, but sufficient amounts are displaced from the steel-concrete interface.
- Installation of physical barrier systems such as coatings, sealers, membranes, and overlays to forestall subsequent Cl– ingress
- A relatively thin zinc surface layer is applied by either hot dipping or electro-deposition. This methodology relies on a relatively low corrosion rate for zinc and its potential for being active to the substrate steel, thereby providing galvanic cathodic protection at defects and penetrations.
- Cathodic prevention is, in effect, identical to cathodic protection, except that it is applied to new, Cl-
-free structures for which current demand is less than for Cl contaminated ones. In addition, the objective here is not to reduce corrosion rate itself (because the reinforcement is passive), but instead to establish a potential gradient that opposes the inward diffusional migration of anions, specifically chlorides. In this regard, the approach functions similarly to ECE, except that, instead of removing chlorides, it retards their entry.
- Concrete mix design modifications involve such factors as reduced w/c, including use of water- reducing admixtures or superplastizers; type of cement; permeability reducing admixtures such as fly ash, silica fume, and blast furnace slag; and corrosion inhibiting admixtures.
- Structural design aspects of corrosion control involve factors such as configurational (geometrical) considerations that minimize or, if possible, eliminate exposure to corrosives.
- Remedies for corrosion-damaged concrete include removal of all delaminated concrete, cleaning of the reinforcement by abrasive blast cleaning, high pressure water, or needle scaling, and use of a concrete patching material.
Conclusions
Common types of corrosion occurring are Pitting, Crevice and Intergrannular corrosion. The two most common causes of reinforcement corrosion are chloride ions and carbonation by atmospheric carbon dioxide.
In wet and cold climates, reinforced concrete for roads, bridges, parking structures and other structures that may be exposed to deicing salt may benefit from use of epoxy-coated, hot dip galvanized or stainless steel rebar, although good design and a well-chosen cement mix may provide sufficient protection for many applications. Epoxy coated rebar can easily be identified by the light green color of its epoxy coating.
Hot dip galvanized rebar may be bright or dull grey depending on length of exposure, and stainless rebar exhibits a typical white metallic sheen that is readily distinguishable from carbon steel reinforcing bar. More techniques like Cathodic protection and ECE are also employed. Use of Fly Ash too delays the effect of chlorides and carbon dioxide.



 Pitting
Pitting Reasons Of Corrosion
Reasons Of Corrosion Loss of Alkanity due to Chlorides
Loss of Alkanity due to Chlorides