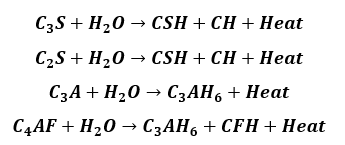In order to activate the adhesive property of cement to allow it to act as a binder, cement has to react with water. When water is added to powdered cement, a chemical reaction is propelled which is very commonly known as hydration of cement.
Cement contains four main compounds of silica and alumina (also called Bogue’s compounds), which when chemically upended with water, produce some hydration products.
These hydrated compounds have the inherent property of binding with each other and with other particles. A number of factors are to be taken into account to affirm that the cement undergoes this crucial process of strength gain in an opportune manner.
Table of Contents
Chemical Reactions
The hydration of cement takes place in several stages, categorized by the rate of heat liberation. Therefore, from the onset of this process to the achievement of a steady-state condition, the amount of heat evolved and its rate of evolution are two variables.
At some stage, the evolution is a quick, incessant process and at the other, it is slow, continued one lasting for days.
When a predetermined quantity of water is mixed with cement, the instantaneous reaction is very fast and a huge amount of heat is evolved. However, this reaction is very quick and lasts for a few minutes, after which the amount of heat starts to dwindle.
Once the rate of heat evolution declines, the nucleation dissolution process is commenced and the hydration products begin to occupy volume inside the cement paste. The following chemical reactions take place,
In the above chemical reactions, CSH stands for calcium silicate hydrate gel also called Tobermorite gel, and calcium hydroxide is abbreviated as CH. The hydrated crystals look like fibrous tubes and are extremely small.
In a fully hydrated Portland cement paste, the Tobermorite gel occupies about 50-60% of net solid volume, whereas the calcium hydroxide crystals (also termed as Portlandite) occupy 20 to 25% of the volume of solids.
The remaining volume of solids is shared by the other hydration compounds given in the chemical reactions above. The CSH gel takes the lead in governing the compressive strength of the cement paste, and its presence is, by all means, advantageous for the cement paste.
On the other hand, calcium hydroxide proves counter-productive for this cause, and its presence proves somehow useless for the paste. Nevertheless, being alkaline in nature, it aids in controlling the pH of the paste. The figure below shows the structure of hydrate gels formed during this process.
Fig.: Structure of Hydrate gels
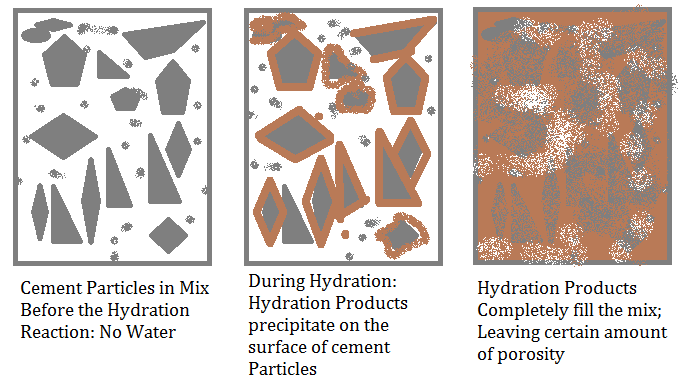
The hydration products begin to accumulate on the outer periphery of the nucleus of hydrated cement particles. This deposition is a slow reaction and takes around 2 to 5 hours. It is called induction or dormant period. As the hydration proceeds, the hydration products slowly begin to deposit on the original cement grains and make it arduous for water to diffuse into the unhydrated nuclei of cement particles.
Consequently, the rate of hydration gets slowed down. The hydration products gradually start occupying the space erstwhile occupied by water. The hydration process ceases when all the water molecules are reacted, or when no unreacted cement particle remains.
Fig.: Summarized hydration process
Heat of Hydration
The hydration of cement is an exothermic process. This means that a considerable amount of heat is evolved in this chemical reaction, which is termed as the heat of hydration. This heat surges the temperature of the paste.
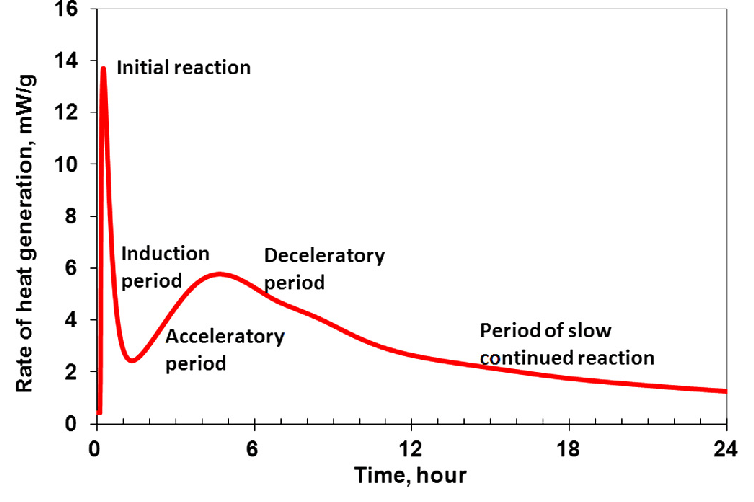
The instant water is added, the temperature suddenly hikes up due to the aluminates reacting with water (initial reaction). However, this temperature surge does not persist for a long period and lasts only a few minutes.
Once the solubility of aluminates is depressed owing to the presence of gypsum, the heat evolution dwindles sharply (induction period).
However, this time the sulphates present in the form of silica begin to start the chemical journey and meanwhile liberate heat that increases the temperature to a relatively lower extreme (acceleration period).
Once the amount of unreacted cement remains less, the heat liberation process begins to decelerate slowly (deceleration period). After a certain time, a steady-state condition is achieved in which very little or no heat is evolved as the hardened cement paste elapses the final set (period of slow, continued reaction).
The graph given below shows the general trend in the variation of rate of heat evolution for ordinary Portland cement.
It can be concluded that different cement compounds hydrate at different rates and evolve different quantities of heat during this process. The rate of heat liberation also depends on the fineness of the cement.
However, the total quantity of heat evolved in a complete hydration process only depends on the relative percentages of Bogue’s compounds present in the cement.
Verbeck and Foster quantified the heat evolution of different compounds and the observations are summarized in the adjoining table.
| Chemical Compound | Heat of Hydration at the Given Age (cal/g) | ||
| 3 days | 90 days | 13 years | |
| Felite (C4AF) | 58 | 104 | 122 |
| Celite (C3A) | 12 | 42 | 59 |
| Alite (C3S) | 212 | 311 | 324 |
| Belite (C2S) | 69 | 98 | 102 |
Given by Verbeck and Foster
The heat of hydration of cement is an additive property. This means that for a complete hydration process, the total heat evolved can be taken equal to the sum of liberated heat at each substage of hydration of a particular compound. Mathematically,
Where A, B, C, and D represent the amount of C3S, C2S, C3A, and C4AF in the cement and a, b, c, and d represent the share of 1% of the corresponding compound to the heat of hydration.
Significance of Hydration Process
The following points highlight the significance of the hydration process:
- It helps the cement to act as a binder, else the powdered cement has no adhesive property of its own. The hydration process accounts for making the cement a gluey substance, activating the adhesion and cohesion processes.
- The hydration process considerably affects the compressive strength of hardened cement.
- The hydration process is to be completely understood to ward off any possibility of thermal cracking in concrete. This is because the very reaction heats the cement paste and can induce thermal cracks if the proportions in the cement are not controlled deliberately.
- The hydration process controls the setting time of cement. Faster is this process, lesser is the time the paste takes to finally set and harden.
Factors Affecting the Hydration Process
Some of the factors that influence the hydration process of cement are listed below:
- The relative proportions of various Bogue’s compounds present in the cement powder affect the hydration process a great deal.
- Environmental factors such as temperature, humidity etc. can also act as an accelerator or retarder for the hydration process. High temperature proves a stimulus for this process and low temperature hinders the same.
- The water-to-cement ratio also affects the cement-water chemical reaction.
- The fineness of cement or the size of cement particles also controls the hydration process. Smaller cement particle indicates more surface area which refers to a greater affinity for water, and hence, hydration will be more.