Table of Contents
What is Portland Cement?
Portland cement is a hydraulic cement (a binder that remains stable under water) that is used in construction works as a cementing or binding material. It is mostly used to make mortar or concrete.
The mortar is a binding glue for stones, bricks, tiles, blocks and all forms of construction units whereas concrete is a heterogeneous mixture of cement, aggregates, and water that has excellent strength in compression.
History and Manufacturing
The word cement is derived from the Roman term “Opus Caementicium” that described some form of masonry made out of crushed rocks with burnt lime as a binder.
In ancient times, the builders were in a constant quest for searching binders that could help them glue together stones or bricks to made structures. The old cementing material used for this purpose was mud, with straw added sometimes to bind dried bricks together. However, it was soon realized that this technique was only fruitful in dry climates and upon contact with water, clay offered no resistance to collapse.
Sometimes, naturally-occurring bitumen was also used to bind stones but that too offered limitations. The first calcareous materials (i.e., compounds of lime) used for cementing purposes were gypsum and lime.
The builders kept travelling the road to discoveries and both the Greeks and the Romans produced hydraulic limes by calcinating limestones. Later on, the Romans used a volcanic ash called pozzolana to form pozzolan-lime mortars because it was found near the village Pozzuoli.
In 1824, Joseph Aspdin, a Leeds builder discovered what we call “Portland cement”. He prepared it by calcining finely ground limestone, mixing it with finely divided clay and calcining the mixture in a kiln until all the carbon dioxide evaporated. Following this, the mixture was finely ground and used as cement.
The name Portland originated as a trade name only and signifies the resemblance of hardened cement with the natural stone in Portland, England and in no way indicates the composition of cement.
The composition of Portland cement can be tailored to achieve different variations suiting varying needs of contemporary construction practices.
Raw Materials of Portland Cement
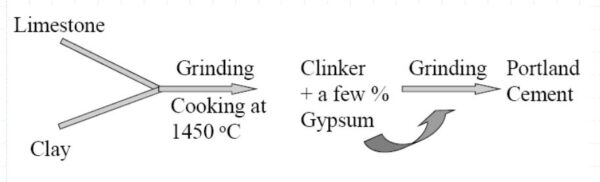
Flowchart showing manufacturing of Portland Cement
The following two types of raw materials are essential to the production of cement.
- Calcareous materials such as limestone or chalk
- Argillaceous materials such as shale or clay

Raw Materials for Portland Cement
The raw materials are ground and mixed in certain proportions depending upon the level of their purity and composition. Following this, they are burned in a kiln at a high temperature of around 1500 C.
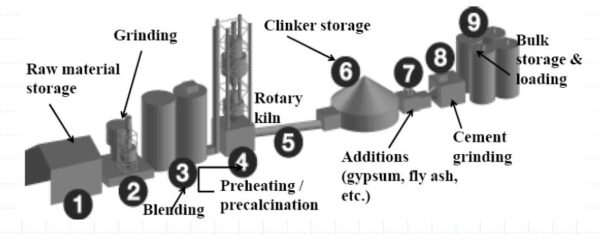
Manufacturing Process
The raw materials fuse and form small nodular-shaped clinkers. The clinkers are cooled and ground to a powdered form. Finally, some gypsum (3 to 5%) is added to regulate the setting time of the final product i.e., the Portland cement.

Cement Clinkers
The manufacturing of cement involves two processes known as the wet process and the dry process, depending upon whether the mixing and grinding of raw materials is done in a wet or dry state.

Cement Manufacturing Plant
Specifications of Cement Bags
Cement is stored and transported in bags. The amount of cement in a bag varies from one country to another, depending upon their local practices.
- Weight of 1 bag of cement =50 kg
- Volume of 1 bag of cement = 0.0345 m3
- Density of cement = 1440 kg/m3
- Specific gravity of cement = 3.15
- Shelf life of a cement bag = 3 months

Stacked Cement Bags
Quality Control of Portland Cement
The stages in the manufacturing of Portland cement need to be closely monitored since they involve complex chemical reactions. Plant chemists are therefore, tasked with analyzing the raw materials fed into the kiln as well as the finished product.
Automated analytical controls have made the monitoring process easy and at-hand. Optimum operating conditions of kilns however, are based on trials and experiences and they require manual supervision.
Composition of Cement
The following are the main constituents of cement and their percentage range present in it.
- Silica or Silicon dioxide (SiO2): clay, sand, calcium silicate, etc.
- Lime or Calcium oxide (CaO): limestone, marble, calcite, seashells, etc.
- Alumina or Aluminium oxide (Al2O3): clay, shale, bauxite, etc.
- Iron oxide (Fe2O3): iron ore, blast furnace dust, etc.
- Magnesium oxide (MgO)
- Sulphur trioxide (SO3)
- Alkalis
From the above-mentioned constituents, the first four i.e., lime, silica, alumina, and iron oxide are responsible for imparting strength characteristics to the cement. On the other hand, the last three ingredients (magnesia and alkalis) are added as impurities to lower the melting temperature and improve combination of lime.
|
Compound |
Percentage |
Notation |
Compound |
Percentage |
|
Lime |
63% |
C |
Sulphur Trioxide |
2 |
|
Silica |
20% |
S |
Alkalis |
1 |
|
Alumina |
6% |
A |
Other |
1 |
|
Iron Oxide |
3% |
F |
Loss on Ignition |
2 |
|
Magnesia |
1% |
M |
Insoluble Residue |
0.5 |
If a greater quantity of lime is present, it will make the cement unsound. By the term “unsound” we mean that the cement will not be able to resist volumetric changes when subjected to temperature gradients. On the other hand, if lime is present in lesser quantity, the strength of the resulting cement will be compromised.
Just like lime, silica is also responsible for development of strength in cement. However, if a large quantity of silica is present in cement, it will also increase its setting time.
The presence of alumina imparts quick setting properties to the cement. Excess alumina lowers the temperature of clinkers during manufacturing of cement, resulting in clinkers not properly burned.
Apart from strength, iron oxide also imparts color and hardness to the cement whereas gypsum is added to regulate the setting time of cement.
The above constituents of cement when come in contact with water, an exothermic reaction is commenced. This reaction is called as “hydration reaction” and as a result of the chemical reactions, 4 compounds are formed that are called “Bogue’s compounds”. These are as follows.
|
Name of Compound |
Oxide Composition |
Notation |
|
Tricalcium Silicate |
3CaO.SiO2 |
C3S |
|
Dicalcium Silicate |
2Cao.SiO2 |
C2S |
|
Tricalcium Aluminate |
3Cao.Al2O3 |
C3A |
|
Tetra-calcium Alumino-ferrite |
4CaO.Al2O3 |
C4AF |
-
Tricalcium silicate or alite (C3S)
It is chemically represented by the formula 3CaO.SiO2 and is present at about 40-70%. This compound provides early strength gain to the cement. 70% of this compound reacts in the first 28 days and facilitates strength development.
-
Dicalcium silicate or belite
The chemical formula of belite is 2CaO.SiO2 and it is present at 20-40 percent. It provides strength to the cement at later stages and therefore, only 30% of it reacts by 28 days.
-
Tricalcium aluminate or celite
The chemical formula of celite is 3Cao.Al2O3 and generates heat in early hydration. It is responsible for flash setting or initial setting of the cement. This compound has little contribution to the strength gaining process. Excess of this compound makes the cement vulnerable to the attack by chemicals especially the sulphate attack.
-
Tetra-calcium Alumino-ferrite or felite
The chemical formula of felite is 4Cao.Al2O3.Fe2O3 and it is present at 1-10 percent. It governs the color of the cement. The presence of iron acts as a flux and facilitates the formation of other compounds.
This compound is also responsible for the initial setting of cement; however, its heat of hydration is lesser than that of tricalcium aluminate. This compound contributes the least in imparting strength to the cement.
The above four compounds define the properties of Portland cement and their relative proportions govern the level of binding that the cement will provide. They control the setting, durability and strength of the cement.
Microstructure of Hydrated Portland Cement
When Portland cement reacts with water, the hydration reaction takes place. The hydration products formed as a result are responsible for the structural integrity of cement at the microscopic level. These hydration products are as follows.
- Calcium Silicate Hydrate (CSH) Gel represents 50 to 60 percent by volume of fully hydrated cement paste. The structure of CSH is not well known but researches have hinted out a layered system. It is mostly amorphous and is very porous.
The presence of CSH gel is what gives strength to the paste, and greater is its quantity in the cement, the better and denser the paste is. On the contrary, cements that do not show appreciable strength do not develop sufficient quantity of CSH.
- Calcium Hydroxide (CH) or Portlandite is the relatively paltry product of cement hydration and is unwanted or superfluous, however, CH is consumed to form CSH and therefore, it not be neglected. It represents 20 to 25 percent by volume of fully hydrated cement paste. CH has a hexagonal morphology which is influenced by temperature and impurities.
- Calcium Sulfo-Aluminate represents about 15 to 20 percent of the volume of hydrated cement paste. It is present in the cement microstructure as hexagonal monosulphate plates.
- Unhydrated cement grains also remain in the cement structure after the hydration reaction is completed. This happens when large cement grains are there in the cement which usually occurs as a result of inefficient grinding of cement clinkers. In addition, a low water-to-cement ratio may also leave some grains unhydrated in the cement because of the formation of denser hydration products around larger grains despite their core remaining unhydrated.
Uses
Initially, the use of cement was confined to making mortars for binding bricks, stones or other construction units. However, later on, Portland cement served as one of the best cementitious materials in the preparation of concrete.

Cement Mortar
Types of Portland Cement as per ASTM C150
The American Standard for Testing and Materials (ASTM) C150 has delineated 8 different types of Portland cement and they are explained herewith. The designation A indicates the imparted property of air-entrainment into the cement whereas MH signifies the property of moderate heat of hydration of a particular cement type.
What differentiates the cement types is their performance when they react with water. Different uses necessitated modifying the constituents of cement to use the best type under a given set of circumstances.
All the given types are manufactured from the same raw materials but have different proportions of constituents and varying physical characteristics.
-
Type I Cement
This cement type is used for general purposes and is the most common among all cement types. Its biggest customer is the ready-mix concrete manufacturers that prepare concrete based on an engineered mix design.
-
Type IA Cement
This cement type is similar to Type I expect that it possesses air-entrained properties. By air-entrainment it is meant that when this type of cement is used in concrete, a network of air bubbles will be created to release concrete of stresses created by freeze-thaw cycles.
-
Type II Cement
The type II cement is very similar to type I expect that it offers moderate sulphate resistance. Sulphate resistance is an important factor to consider in areas where the concrete is susceptible to the attack by sulphates as in case of concrete directly exposed to sea water or soil containing sulphates.
-
Type IIA Cement
This type of cement is a slight modification of the Type II cement. It offers an additional feature of air-entrainment and can be a good choice where moderate sulphate resistance along with resistance to free-thaw is required.
-
Type II (MH) Cement
This cement type is also a modified version of the Type II cement. It has an additional feature of exuding low heat of hydration. The exothermic hydration reaction may sometimes generate massive amount of heat that may speed up the hardened of concrete. In such circumstances, the amount of heat of hydration ought to be controlled.
-
Type II (MH)A Cement
This type of cement has the combined features of Type II(MH) and Type IIA. It provides air-entrainment and also reduces the heat of hydration of cement.
-
Type III Cement
This type of cement is called high early strength cement since it acts a catalyzes the setting of cement. It can effectively be used in precast members, during cold weather, in roadway applications or in repair work. All these construction activities require speeding up the strength gaining process.
-
Type IIIA Cement
This type of cement is a modification of the Type III cement and offers air-entrainment properties to the final product.
-
Type IV Cement
This is the low heat of hydration cement since it delays the hydration reaction as a result of which lesser heat is generated. However, this cement type is no longer common since other viable options are now available to control the heat of hydration.
-
Type V Cement
This type is the sulphate-resistant cement and if offers high resistance to the attack by sulphates. It can be used in areas where concrete is exposed to seawater, industrial sewage, or sulphate-containing soils and also is concrete pipes that carry raw sewage. This type of cement also has a lower heat of hydration.
Field Tests on Portland Cement
The suitability and quality of cement can be assessed in the field through the following field tests.
-
Adulteration Test
This test includes taking cement in your hand and rubbing it between the fingers. If the rubbing gives a smooth feeling, the cement is of a good quality and if it feels gritty it is not.
-
Color Test
The color of cement can also be an indication of how fine the preparation and grinding works have been carried out in the kiln. If the cement in bag has a uniform greenish grey color, it is of a good quality.
-
Float Test
A good cement floats on water and therefore, if you thrust a handful of cement in a bucket of water, it should float.
-
Temperature Test
If you insert your hand in a bag containing cement, it should feel cool.
-
Checking Date of Packing
The cement bags have printed on them the date of their packaging. From that date onwards, the cement remains in a good state till 3 months. Therefore, prior to opening up a cement bag and using it, you must check the date of packing. If the time has exceeded 3 months with reference to that date, the bag must not be used.
The above tests are crude filed practices and are not very didactic of the cement’s strength characteristics. In case any information is required on how a particular cement type will perform under specific field conditions, the above field tests must not be entirely relied upon and comprehensive lab testing is suggested in such cases.
Lab Tests on Cement
The properties of cement in the fresh state influence its placing and workability whereas its hardened properties influence its strength and durability.
-
Compressive Strength Test
The compressive strength of cement is determined by preparing cube samples from cement-sand mortar. 1 part cement to 2.75 parts sand is used to make the mortar which is then cast into 50mm cubes which are kept for 1 day and then tested for compression.
The code has specified the strength values at varying ages that the cement must satisfy.
-
Setting Time Test
The setting time of cement can be determined using Vicat apparatus with a penetration needle. The determination of setting time is important to prevent flash setting and false setting of cement.
The initial setting time of ordinary Portland cement varies from 1 to 4 hours and the final setting time varies from 3 to 6 hours.
-
Soundness Test
The resistance of cement to volumetric changes can be determined using Le Chatelier’s apparatus. The causes of unsoundness in cement are excess of hard burned free lime or magnesia.
-
Determination of Bulk Density of Cement
The bulk density of cement is determined using Le Chatelier’s Flask and it varies between 830 kg/m3 (52 lb./ft3) and 1650 kg/m3 (103 lb./ft3).
-
Chemical Tests
Chemical tests on Portland cement aim at determining different oxide compositions. This test can be used to calculate the potential compound composition of C3S, C2S, C3A, C4AF.
-
Fineness Test
The fineness of cement can be determined by either measuring the time to pass air through a porous bed of cement sample (Blaine’s Test) or passing light through a suspension of cement particles and measuring the intensity of light (Wagner’s Test).
In either of the above tests, the fineness is measured in units of area per unit mass (m2/kg). Todays’ cements have a fineness value of 370 m2/kg.
Finer is the cement, faster is the hydration process and more amount of heat is generated resulting in higher early-age strength and lesser long-term gains.
-
Heat of Hydration Test
This test is used to measure the heat generated from cement as it hydrates.
-
Air Content Test
This test is used to determine the air content of mortar from a unit weight measurement. The significance of this test is that it helps ensure that the cement does not entrain undesired air.
-
Consistency Test
The consistency of cement can be determined by checking the flowability of cement mortar on a flow table.
Benefits in Construction
- In most types of construction works, a binding material is required to glue together construction units in order to get structural integrity. Cement is one such excellent binding material that works efficiently and provides great strength under all circumstances.
- The use of cement as a confining material in concrete has taken the construction industry to a whole new level. Reinforced Cement Concrete (RCC) provides excellent strength properties and can be molded to any shape.
- As a binding material, cement is easily available in local markets across the country.
- Cement is a good fire-resisting material and this property of cement helps a lot in safety of structures.
How to Check if Your Cement is of Good Quality?
Physically, the rubrics of judging cement are crude and must not be entirely relied upon. However, they can serve as a judgement and can be used to get an idea about the cement being used. In this regard, the following physical characteristics can help define a good quality cement.
- Its color should be greenish grey.
- It should feel smooth when touched by hands.
- If you take a handful of cement and thrust it in water, it should sink slowly and not instantaneously all at a time.
- If you put your hands in a bag of cement, it should feel cold.
- The cement should be free from lumps.
World Production of Portland Cement
Annually, around 500 million tonnes of Portland cement are produced in the world. Out of the total quantity produced per year, the percentage use in concrete is 7 to 96% depending upon the location.
Some countries do not entirely rely on cement as the binding agent for their construction work, they go for other alternatives.
Drawbacks of Using Portland Cement
Although cement forms the backbone of the construction industry in essentially every country around the world, its use also has drawbacks a few of which are discussed here.
- The cement industry accounts for industrial air pollution in the form of harmful gases such as sulphur dioxide, carbon monoxide and oxides of nitrogen. All of these can be very harmful to the health of humans and other living things in our ecosystem.
- Cement manufacturing is also increasing the carbon footprints, contributing a significant amount in the greenhouse gas emissions.
Frequently Asked Questions (FAQs)
Why is Portland cement named so?
When Joseph Aspdin discovered cement, it was found to resemble the color of natural limestone that was quarried on the Isle of Portland. Portland was a peninsula on the English Channel and owing to color resemblance, it is given the name Portland cement.
What is setting time of cement?
The setting time of cement is the time between the instant water is added to the dry cement and the time where the paste shows some stiffness. After setting, the cement paste begins to harden and loses all of its plasticity gradually.
What is the difference between flash setting and false setting of cement?
The flash setting of cement means very rapid setting of the paste with significant liberation of heat of hydration. It usually results when not enough quantity of gypsum is present in the cement. Flash set cannot be remixed and the paste is to be discarded in such a case.
On the other hand, false set means rapid setting without much liberation of heat of hydration. It occurs when too much gypsum is present in the cement. If a false set occurs, the plasticity of the cement paste can usually be recovered by adding some additional quantity of water and remixing the paste.







