Table of Contents
Wastewater Treatment

| Composition of untreated domestic wastewater | ||
| Constituent | Abbreviation | Concentration (mg/L) |
| Biological oxygen demand | BOD | 100 – 350 |
| Chemical oxygen demand | COD | 250 – 1000 |
| Total dissolved solids | TDS | 250 – 1000 |
| Suspended solids | SS | 100 – 400 |
| Total Kjeldahl Nitrogen | TKN | 20 – 80 |
| Total phosphorus | TP | 5 – 20 |
Municipal Wastewater Treatment Systems
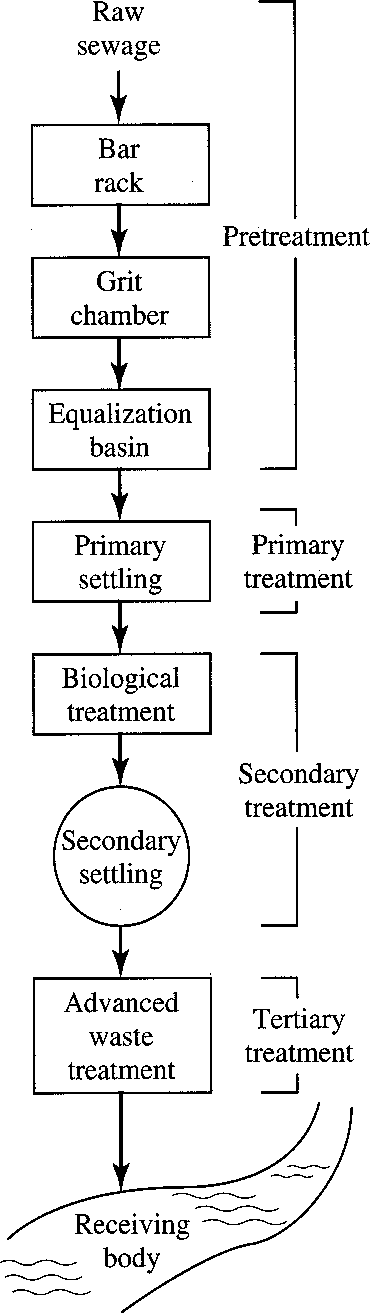
- Preliminary treatment – Removes materials that can cause operational problems, equalization optional
- Primary treatment – Remove ~60% of suspended solids and ~35% of BOD
- Secondary treatment – Remove ~85% of BOD and suspended solids
- Advanced treatment – varies: 95+ % of BOD and solids, N, P
A) Preliminary Treatment
1) Screening

There are three different types of screens that may be used, trash racks, manually cleaned racks, and mechanically cleaned racks. Two channels are provided to allow one to be taken out of service for cleaning and maintenance.
- Coarse screens remove large solids, rags, and debris from wastewater, and typically have openings of 6 mm (0.25 in) or larger.
- Fine screens (1.5-6mm) are typically used to remove material that may create operation and maintenance problems in downstream processes, particularly in systems that lack primary treatment.
- Very fine screens (0.2-1.5mm) have openings of 0.2 to 1.5 mm (0.01 to 0.06 in) and are placed after coarse or fine screens can reduce suspended solids to levels near those achieved by primary clarification.
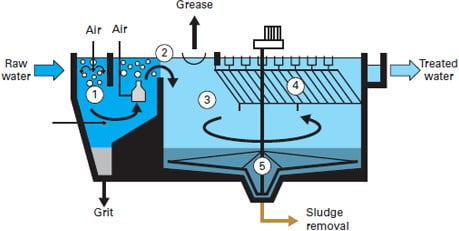
2) Grit Chambers
Grit is the term used for small, but dense material such as sand, dirt, or broken glass. If not removed separately grit can cause wear and damage to mechanical devices in a treatment plant. There are several methods to remove grit, though the most common is to send it through a channel where the speed of the water is such that the grit settles and can be removed, while the rest of the water can flow on to further treatment. Like the bar screen channels, there are always two of these channels, allowing for one to be cleaned or repaired while the other remains in use.
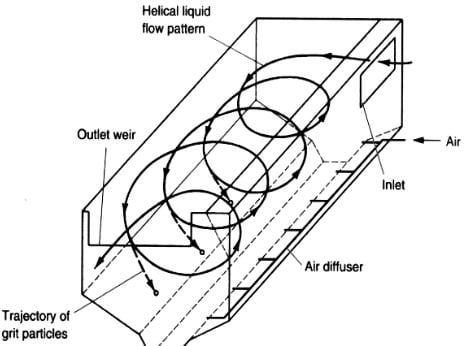
There are two common types of grit removal devices: velocity controlled and aerated.
- Velocity Controlled The system is a long narrow sedimentation basin with better control of velocity (though weir). It has hopper at the bottom where grit accumulates. It can be cleaned mechanically and manually.
- Aerated Grit Chambers Rectangular tank with aeration by coarse bubble diffuses along one side creates spiral flow. Removal size selection is done by controlling the air and in turn the velocity.
B) Primary Treatment
The objective of primary treatment is the removal of settleable organic and inorganic solids by sedimentation, and the removal of materials that will float (scum) by skimming. Approximately 25 to 50% of the incoming biochemical oxygen demand (BOD5), 50 to 70% of the total suspended solids (SS), and 65% of the oil and grease are removed during primary treatment.
Some organic nitrogen, organic phosphorus, and heavy metals associated with solids are also removed during primary sedimentation but colloidal and dissolved constituents are not affected. The effluent from primary sedimentation units is referred to as primary effluent.
1) Sedimentation
- The process is achieved through sedimentation tanks and is gravity based. Primary sedimentation tanks or clarifiers may be round or rectangular basins, typically 3 to 5 m deep, with hydraulic retention time between 2 and 3 hours.
- Settled solids (primary sludge) are normally removed from the bottom of tanks by sludge rakes that scrape the sludge to a central well from which it is pumped to sludge processing units. Scum is swept across the tank surface by water jets or mechanical means from which it is also pumped to sludge processing units.
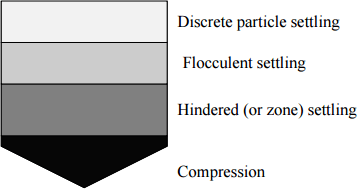
Settling properties of particles are often characterized into four types depending upon particle concentration and interaction:
- Type I: settling applies to particles that settle discretely with constant velocity. They don’t flocculate or stick e.g. sand and grit material
- Type II: In type II particles flocculate during settling. As flocculating, size is continuously changing, therefore, velocity also changes. It generally increases e.g. alum or iron coagulation
- Type III: In type III particles concentration increases with depth, zone settling occurs. Particle concentration are higher than 1000 mg/L such that particles tend to settle as a mass thus forming a distinct clear zone and sludge zone.
- Type IV: This occurs when the particle concentration is so high that so that particles at one level are mechanically influenced by particles on lower levels. The settling velocity then drastically reduces.
Settling phenomena in Sedimentation Tank
Strokes Law (Type I Settling)
C) Secondary Treatment
1) Dispersed/suspended Growth
- Activated sludge
- Membrane bioreactors
- Aerated lagoons, stabilization ponds
2) Fixed/attached Growth
- Trickling filters
- Rotating Biological Contactors (RBCs)
3) Activated Sludge Process
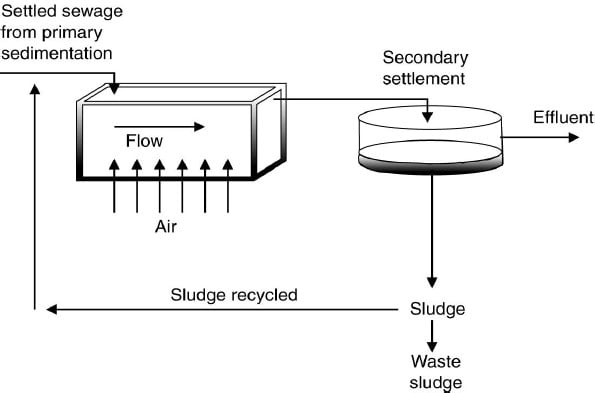
Process in which a mixture of wastewater and microorganisms (biological sludge) is agitated and aerated. It leads to oxidation of dissolved organics. After oxidation, sludge is separated from wastewater.
To induce microbial growth, we need:
- Food (organics) and oxygen
- Mixed Liquor Suspended Solids (MLSS) of 3,000 to 6,000 mg/L
td = approximately 6 – 8 hr
8 m3 of air provided per m3 of wastewater treated
- Air is injected near bottom of aeration tanks for mixing through system of diffusers.
- MLSS and F/M controlled by wasting a portion of microorganisms.
- As the microorganisms grow and are mixed by the agitation of the air, the individual organisms clump together to form an active mass of microbes called activated sludge.
- Moat of the settled sludge is returned to the aeration tank to maintain high population of microbes to permit rapid breakdown of the organics called return sludge.
- The mean cell residence time (θ) also called solid retention time (SRT) or sludge age, is defined as the average amount of time that microorganisms are kept in the system.
- Mixed Liquor is a mixture of raw or settled wastewater and activated sludge within an aeration tank in the activated sludge process. Mixed Liquor Suspended Solids (MLSS) is the concentration of suspended solids in the mixed liquor, usually expressed in milligrams per litre (mg/l).
F/M ratio- Major design parameter
F/M (low rate of wasting)
- starved organisms
- more complete degradation larger, more costly aeration tanks more O2 required
- higher power costs (to supply O2)
- less sludge to handle
High F/M (high rate of wasting)
- organisms saturated with food
- low treatment efficiency
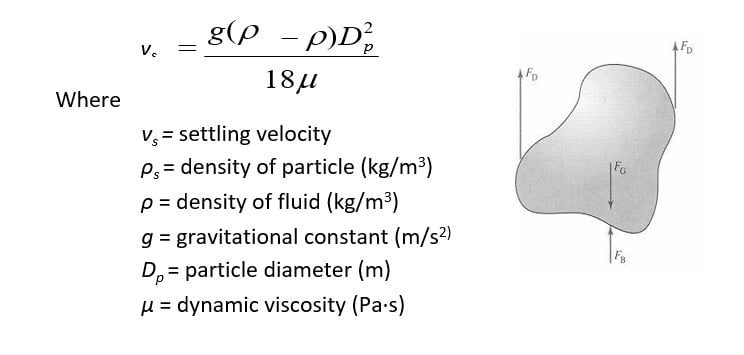
4) Membrane bioreactors (MBR)
- Also known as membrane biological reactors
- MBRs decrease the size of secondary treatment tankage and improve TSS
- separation efficiency
- They are submerged in the aeration tank. They draw water from mixed liquor into hollow fiber membranes submerged in activated sludge aeration tank.
- Thus they avoid need for secondary clarifier.
- Microfiltration pores have a size of about 0.2, so they are very effective in TSS removal. As they retain most of the TSS, some activated sludge must be continuously bled off through Waste Activated Sludge line to maintain desired MLSS concentration.
Membrane bioreactors (MBR)
Membrane bioreactors (MBR)
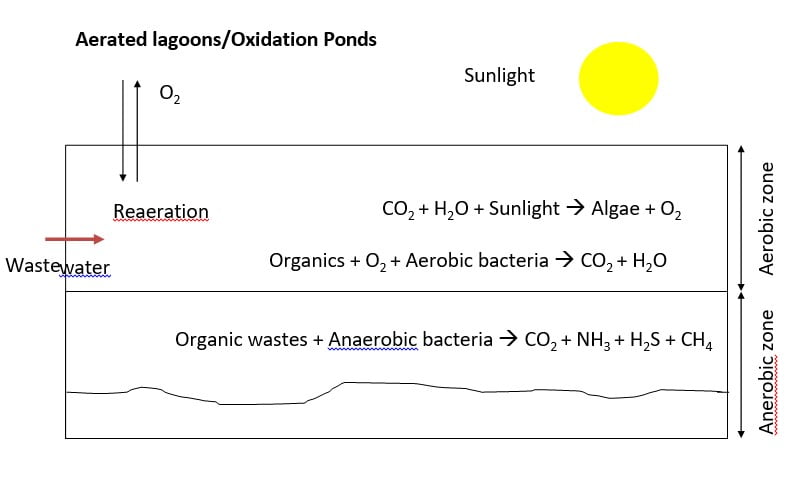
5) Aerated lagoons/Oxidation Ponds
- “Oxidation Pond” is large shallow pond typically 1 to 2 m deep where raw or
- primary treated sewage is decomposed by microorganisms.
- Conditions are similar to a eutrophic lake. Decomposition near the surface is aerobic, whereas near the bottom is anaerobic.
- “Facultative ponds” are those which have both aerobic and anaerobic zones.
- Deeper ponds or “lagoons” are mechanically aerated.
- Aerobic ponds are shallow ponds with depth less than 1 m. Such ponds develop intense algal growth. Dissolved oxygen is maintained throughout the entire depth mainly by photosynthesis.
- Anaerobic ponds are used as pretreatment of high strength wastes with BOD load of 400-3000 kg/ha.day. Such ponds are constructed with a depth of 2.5- 5m as light penetration is unimportant.
- Facultative pond functions aerobically at the surface while anaerobic conditions prevail at the bottom. They are often about 1 to 2 m in depth. The aerobic upper zone is maintained by photosynthesis and surface reaeration.
- Maturation ponds used for polishing effluents from other biological processes. Dissolved oxygen is maintained by photosynthesis and surface reaeration. Also known as polishing pond.
- A possible sequence of ponds for the treatment of waste. Strong wastes with a BOD over 300 mg/l are usually treated in an anaerobic pond followed by treatment in a facultative pond with final polishing in a maturation pond.
- Settled sewage can be run directly into the secondary stage, the facultative pond.

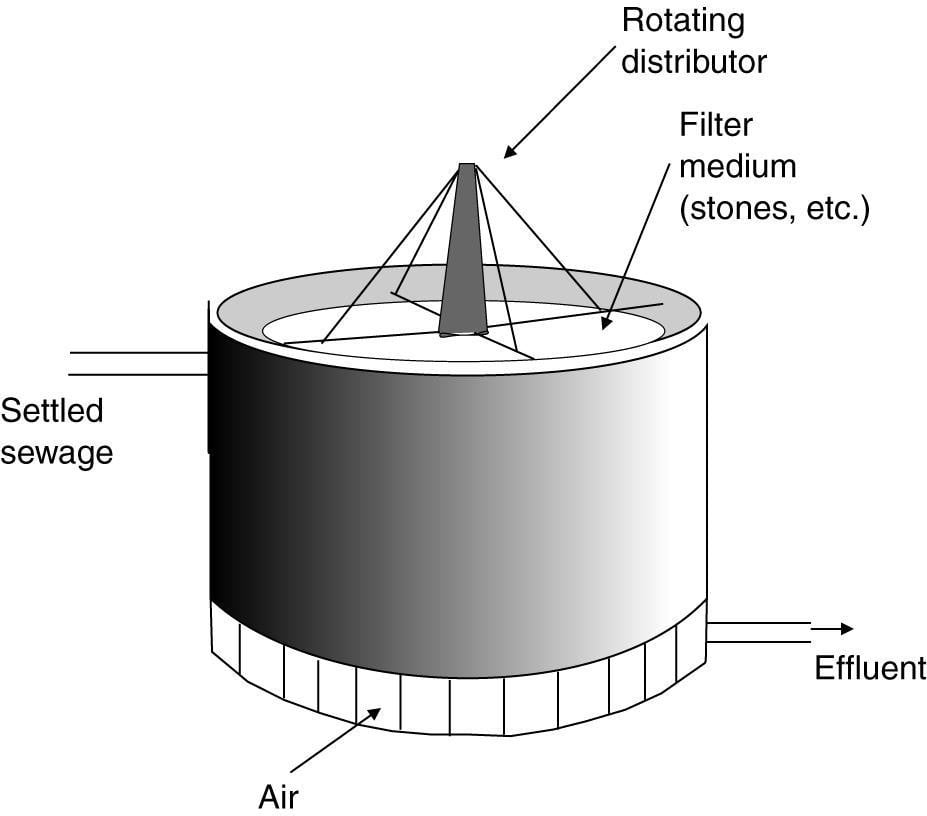
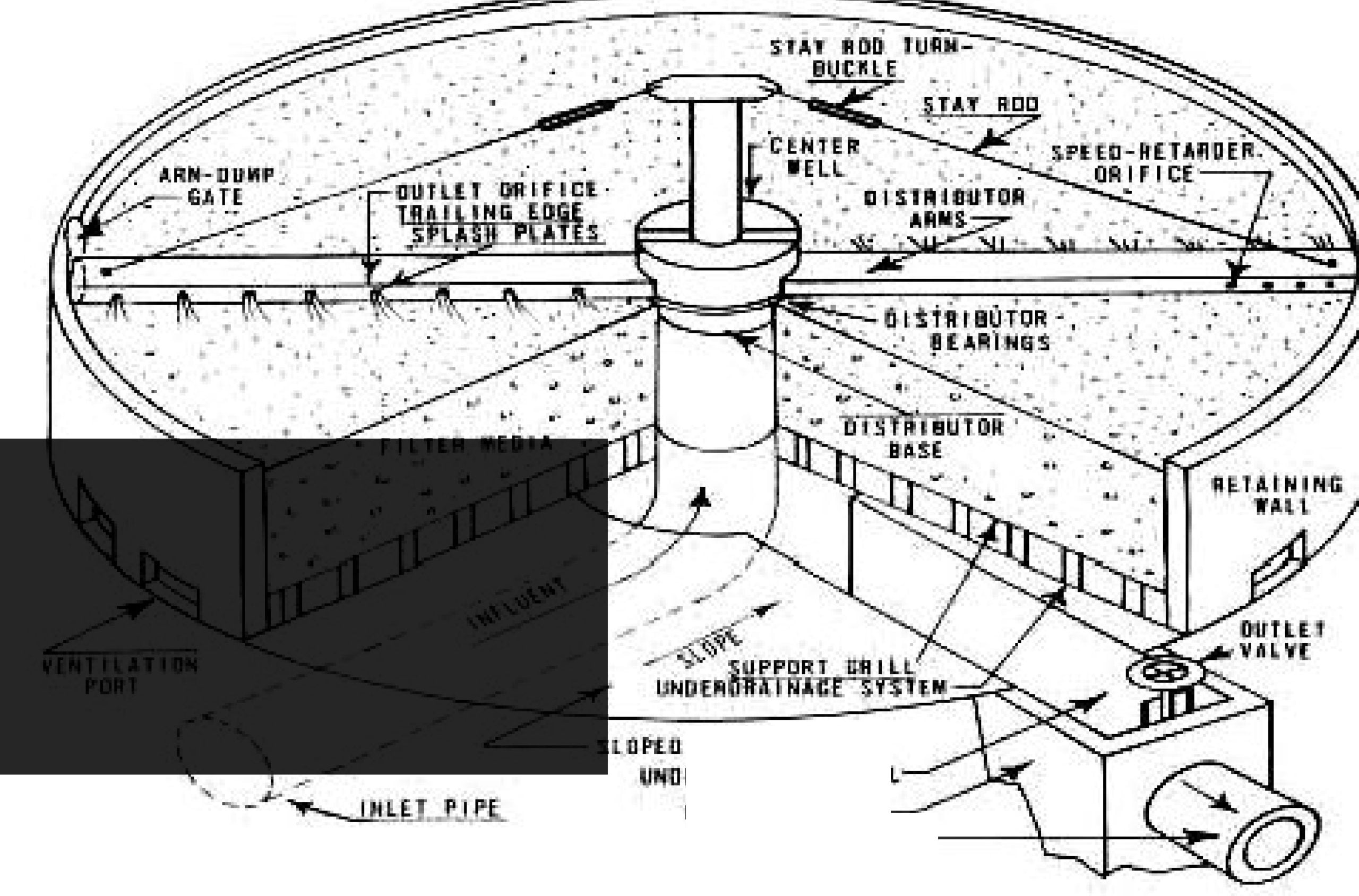
6) Trickling Filters
The design of a trickling filter. The circular vessel 1-3 m in depth is filled with stones, clinker or slag and settled sewage is run from the rotating arms on to the bed. The organic material in the sewage is metabolized by the microbial biofilm, which develops on the stones. The clean effluent is collected at the bottom of the filter. Circulation of air through the filter bed is encouraged by air vents at the base of the vessel.
Trickling filter
7) Rotating Biological Contactors (RBC’s)
- Consists of series of closely spaced discs mounted on a horizontal shaft and rotated while ~40% of each disc is submerged in wastewater.
- RBC’s is an extremely efficient secondary biological wastewater treatment system.
- It utilizes aerobic fixed film technology to reduce organic matter (BOD), total suspended solids (TSS), ammonia and phosphorous from domestic and industrial wastewater, achieving a high quality final effluent that is safe for discharge. Know more about how modern plants treat industrial wastewater.
8) Land Application
- A variety of methods exist for land application of wastewater bio-solids. Land treatment involves applying the wastewater to land by one of the several conventional irrigation techniques. Treatment is provided by the natural processes as the wastewater moves through the plant and soil system.
- Slow rate process Wastewater is applied to the vegetated land as irrigation water for the crops. Wastewater can be applied by sprinklers or by flooding the land. Wastewater is evapo-transpirated i.e. used by plants and percolates through soil.
- Rapid infiltration system Wastewater is applied to relatively permeable soil within a basin at a much higher rate. Treatment is accomplished by natural processes in the soil. Requires much smaller land areas and recharges the ground water table. Plants may be present but play only a minor role.
9) Overland flow process
Wastewater is applied to sloping field. Treatment is provided by the plants and the microbial biomass at the soil surface as the wastewater flows down a sloping field. The process minimizes percolation into the soil and renovated water is collected at the bottom of the slope and discharged into a surface water body.
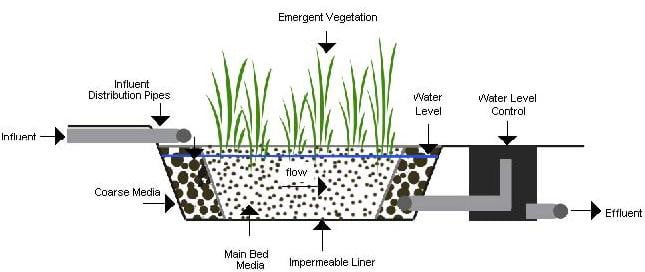
10) Wetland treatment system
Wastewater is applied to natural and artificial wetlands. Renovation is accomplished by sedimentation and biological activity of the plants and microorganisms present.
D) Tertiary Wastewater Treatment
- Phosphorous Removal
- Nitrogen Control
- Filtration
- Carbon Adsorption
1) Removal of Nitrogen and Phosphorus from Wastewater
- Sewage also contains nitrogen- and phosphorus-containing compounds. The nitrogen-containing compounds in sewage are ammonia, proteins and amino acids. Increasingly nitrates are being found in wastewater as a result of run- off from agricultural land. Ammonia is formed from urea and is poisonous to aquatic life at concentrations as low as 0.5 mg/l.
- Nitrogen compounds can be removed by physical, chemical and biological treatment. Despite the number of chemical and physical methods, increasingly biological methods are used to remove nitrogen.
- Ammonia is oxidized rapidly to nitrate in a process known as nitrification. The conversion is carried out by two groups of chemoautotrophic bacteria, which use the oxidation of ammonia as a source of energy.
- In the first stage nitrite is formed and the growth of these bacteria (e.g. Nitrosomonas, Nitrosococcus) is very slow (the release of hydrogen ions leads to a drop of the pH). The nitrite formed is converted to nitrate by other bacteria (e.g. Nitrobacter). As the oxidation of nitrite to nitrate yields less energy than the oxidation of ammonia, the growth rates of the latter bacteria are also very slow. Recent bioaugmentation with nitrifying bacteria has been shown to be effective in maintaining nitrification in stress situations such as low temperatures and high sludge residence times.
2) Denitrification
- Nitrification combined with the nitrates from agricultural run-off can give rise to high nitrate levels (above 50 mg/l) in waterways, which are used to supply drinking water. High nitrate levels are associated with methaemoglobinaemia, which affects children below the age of 6 months. A biological process of nitrate removal is denitrification.
- The biological conversion of nitrate to nitrite and eventually nitrogen occurs under conditions where oxygen is very low or absent. When oxygen levels are low inorganic ions such as nitrate, phosphate, and sulphate can act as electron acceptors and will be reduced.
- A number of facultative heterotrophic micro-organisms occur in sewage- treatment systems which are capable of converting nitrate to nitrogen provided an electron donor is present. The electron donor is usually an organic compound and in some cases methanol has been used to supplement the normal organic source.
- The process of dentrification requires low oxygen levels (i.e. is anaerobic), an organic carbon energy source, a level of nitrate of 2 mg/l or above, and a pH of 6.5-7.5. Phosphorous removal through chemical precipitation uses compounds such as Alum, Ferric Chloride or Lime.
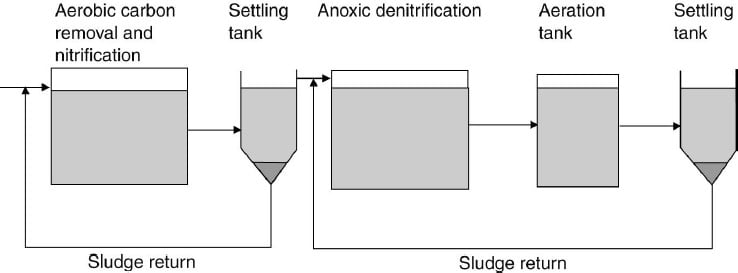
- An outline of a two-stage process for both nitrification and denitrification. In the first stage the normal activated-sludge process occurs with the removal of organic materials and nitrification. In the second stage anoxic conditions cause denitrification which is followed by an aeration tank to strip out the nitrogen formed to ensure precipitation in the settling tank.
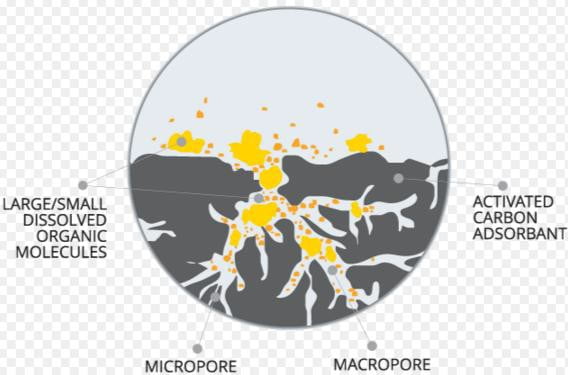
3) Carbon Adsorption
Even after secondary treatment, the soluble organic materials that are resistant to biological breakdown will persist. Such materials are often referred to as Refractory organics.
- The most practical available method for removing refractory organics is
- by adsorbing them on activated carbon.
- Adsorption is accumulation of materials at an interface. In wastewater treatment case, the liquid/solid boundary layer act as interface. Accumulation results due the physical binding of the molecules at the solid surface.
- Carbon is activated by heating in the absence of oxygen, thus forming pores of different sizes within carbon.
- After adsorption capacity of the carbon has been exhausted, it can be restored by heating it in a furnace at a sufficiently high temperature. This drives off the adsorbed organics via thermal damaging.
- Oxygen levels are kept low so as to avoid carbon burning.