Table of Contents
Introduction:
This experiment basically relates the amount of saturation of oxygen in the given water sample such as that of drinking purpose, industrial use, or supplies. Water is one of the most vital component of living whereas its major purification parameter is the amount of oxygen dissolved in it.
Both the excess and loss of oxygen are highly affective in terms of water. A huge amount of oxygen in water may turn the process of increased oxidation which has adverse affects in compliance to normal level. Therefore this factor of ang water component is to be determined properly to avoid any unconformity.
Related theory:
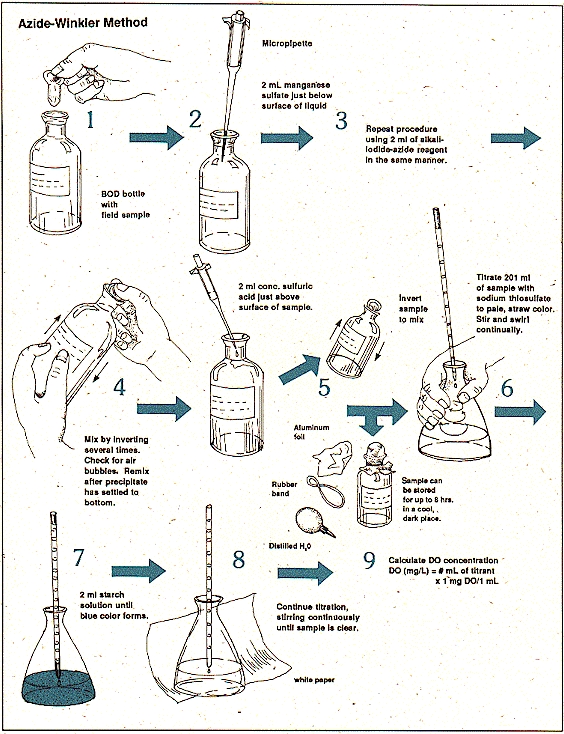
Figure 1 winkers method
This experiment being specific is based on the principles of Winkler’s method being modified also termed as azide modification. the different properties of water i.e. biochemical, physical, or chemical activities of the water sample determine the amount of oxygen being dissolved in the sample.
The major pollution or waste treatment tool is the D.O. determination. The improvements obtained through different methods and the techniques adopted so far for its determination concludes that the most adequate and accurate method obtained so far is this.
This test is basically carried out on the basis of divalent magnesium added in the solution form along with a strong alkali addition in the sample. This results into the oxidation of the solution of manganese into a divalent hydroxide with a valency of greater states. If the ions of iodide are present this is reverted into a divalent state along with the liberation of the iodine equal to the dissolved oxygen original content and is then titrated through thiosulphate standard solution.
Apparatus:
The following apparatus and reagents are adopted for this experiment:
- A bottle with 300mL capacity and a stopper.
- A burette.
- A set of pipettes.
- A solution of manganous sulphate.
- An alkali-based reagent iodide azide.
- An indicator i.e. starch.
- A standard solution of sodium thiosulphate. (0.025N)
- A concentrated solution of sulphuric acid. (36N)
- A standard solution of potassium dichromate. (0.025N)
Procedure:
- 2mL of both the solutions manganese and iodide are added in the sample to be observed i.e. 300mL in a container. The added reagents should be added by dipping the pipette inside the solution properly.
- The stopper is now provided with proper care and adequacy with the proper removal of air bubbles through it can be made sure by at least inverting of 15 times.
- The sample is again subjected to shaking when the settlement of the precipitation takes place with the deposition of a clear floc on the surface of manganese hydroxide.
- The stopper is removed carefully after 2 minute of the settling .
- Now add 3mLof sulphuric acid solution (conc.) running down through the neck of the bottle properly.
- The bottle is again covered by the stopper and gently mixed until the process of dissolution completes.
- A solution of 203mL is measured and added shifted to an Erlenmeyer flask. The quantity proportioned now is 200mL as mL of both the solutions are already added before so is given by:
= (200300)/(300-4) = 203mL
- This solution is now titrated against 0.25N solution of sodium thiosulphate into a pale color (straw).
- The starch solution of 1-2mL is now added and the titration is carried out for the (first) disappearance of the solution color by noting the volume of the sample adopted for the titration process which provides the exact amount of D.O. directly.
Observations:
| Sample# | Trial# | Volume of Sample (mL) | Burette reading | Titrant volume (mL) | D.O. in (mg/L) | |
| initial | final | |||||
| Sample #1 | —– | —– | —– | —– | —– | —– |
| —– | —– | —– | —– | —– | —– | |
| —– | —– | —– | —– | —– | —– | |
| Sample #2 | —– | —– | —– | —– | —– | —– |
| —– | —– | —– | —– | —– | —– | |
| —– | —– | —– | —– | —– | —– | |
| Sample #3 | —– | —– | —– | —– | —– | —– |
| —– | —– | —– | —– | —– | —– | |
| —– | —– | —– | —– | —– | —– | |
Calculations:
| Sample description | D.O. mg/L |
| Sample #1 | —– |
| Sample #2 | —– |
| Sample #3 | —– |
Mean D.O. = ——— mg/L
Comments:
Its major applications are the cleanliness of health of lake or stream. The support to a specific amount of biomass along with the decomposition of the sample. The following table is however set as a standard for the solubility of the oxygen sample.
| Temperature () | Solubility of oxygen (mg/L) |
| 0 | 14.6 |
| 5 | 12.8 |
| 10 | 11.3 |
| 15 | 10.2 |
| 20 | 9.2 |
| 25 | 8.6 |
| 100 | 0 |
Precautions:
Following are the precautions adopted:
- The contamination of the sample is highly prevalent.
- The pipette should be properly filled.
- The sample should be poured in proper measured amount.
- The titration is to be carried out with proper care and adequacy.
- The lid is to be maintained properly.