Table of Contents
Water pollutants
- Water that has been withdrawn, used and then returned will be polluted in one way or another.
- Agricultural return water contains pesticides, fertilizers and salts
- Municipal return water contains human sewage, pharmaceutical waste and surfactants
- Power plants discharge water with elevated temperatures
- Besides this, water comes from natural sources such as groundwater reservoirs, from where it acquires pollutants such as fluoride, arsenic and antimony
- Much of the mercury in water is deposited from air after it is emitted from coal power plants
Criteria for pollutant classification
-
- Substance’s environmental persistence
- Relative toxicity
- Deleterious impact (on humans, plants and animals)
- Occurrence frequency
- Concentration
- Immediacy of impact
Sources of water pollution
- Point sources:
- Factories, power plants, sewage treatment plants, underground coal mines, and oil wells are classified as point sources because they discharge pollution from specific locations, such as drain pipes, ditches, or sewer outfalls.
- These sources are discrete and identifiable, so they are relatively easy to monitor and regulate.
- It is generally possible to divert effluent from the waste streams of these sources and treat it before it enters the environment.
- Nonpoint sources:
- In contrast, nonpoint sources of water pollution are scattered or diffuse, having no specific location where they discharge into a particular body of water.
- Nonpoint sources include runoff from farm fields and feedlots, golf courses, lawns and gardens, construction sites, logging areas, roads, streets, and parking lots
- nonpoint sources are often highly episodic.
- nonpoint pollution is atmospheric deposition of contaminants carried by air currents and precipitated into watersheds or directly onto surface waters as rain, snow, or dry particles.
List of pollutants
- The list of water pollutants is lengthy so it has been categorized in various major categories:
- Pathogens
- Oxygen-demanding wastes
- Biochemical oxygen demand
- Nutrients
- Salts
- Thermal pollutants
- Heavy metals
- Pesticides
- Volatile organic compounds
Pathogens
- Pathogens are disease-causing organisms that live and grow within the host
- This resulting growth in a host is called infection.
- Examples of pathogens associated with water include bacteria, viruses, protozoa and helminths (parasitic worms)
- Human waste contamination is the major cause of pathogens entering
- Infectious disease epidemics usually take place in crowded areas with poor sanitary
- If epidemics become global in proportion, they are termed pandemic.
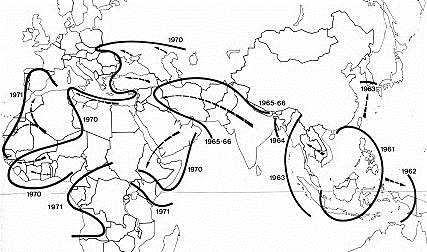
Cholera pandemic 1961
Types of water related diseases
- Water-borne diseases
- Spread by ingestion of contaminated water
- Examples: cholera and typhoid
- Water-washed diseases
- Associated with lack of sufficient water to maintain cleanliness
- Examples: trachoma and scabia
- Water-based diseases
- Involve water contact but don’t require ingestion
- Examples: schistosomiasis and dracunculiasis
- Water-related diseases
- Involve a host that depends on water for its habitat
- Examples: Malaria and dengue
Oxygen-demanding wastes
- Dissolved Oxygen (DO) is one of the most important measures of water quality
- Saturated value of DO in water is modest (8 – 15 mg/L)
- Oxygen-demanding wastes are substances that oxidize in the receiving body of They utilize DO, which reduces the amount available to aquatic species.
- In addition, as DO levels fall, undesirable odors, tastes and colors reduce acceptability of water for human
- These pollutants are usually biodegradable waste from industry but may also include inorganic substances
Biochemical oxygen demand (BOD)
- When biodegradable organic matter is released into water body, microorganisms decompose
- Aerobic decomposition
- Anaerobic decomposition
- Methane emitted from bodies of water is also called swamp gas.
- Amount of oxygen required by microorganisms to oxidize organic waste aerobically is called biological oxygen demand (BOD).
- Often expressed as mg of O2 required per liter of wastewater (mg/L)
- Made up of carbonaceous oxygen demand (CBOD) and nitrogenous oxygen demand (NBOD)
Nutrients
- Nutrients are chemicals essential for growth of living things.
- Common nutrients are: nitrogen, phosphorus, carbon, sulfur, calcium, potassium, iron, manganese, boron and cobalt.
- Nutrients can be considered water pollutants when their concentration is sufficient to allow excessive growth of aquatic plants, particularly algae.
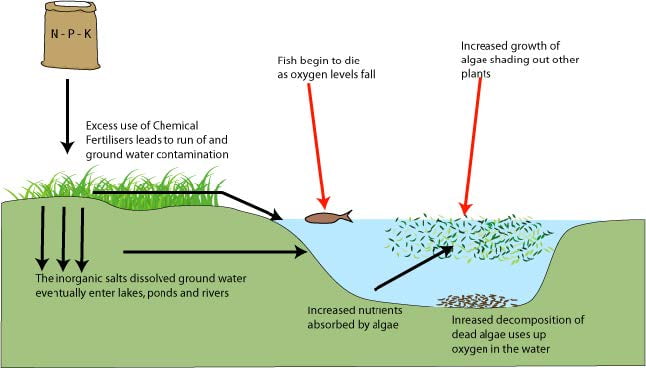
- Nutrient enrichments can lead to algal blooms.
- Decomposition of these algal blooms removes oxygen from the water.
- The process of nutrient enrichment is called eutrophication.
- The nutrient that is least available to the plant is called limiting nutrient.
- In general, seawater is most often limited by nitrogen, and freshwater lakes are most often limited by phosphorus.
- Major sources of nitrogen: municipal wastewater, runoff from agricultural farms, chemical fertilizers and nitrogen deposition from atmosphere
- Nitrogen in the form of nitrate (NO3) is a serious public health threat.
- Certain bacteria convert nitrate to nitrite (NO2) which oxidized hemoglobin in the blood stream and renders it incapable of carrying oxygen (usually occurs in infans – blue baby syndrome)
- Major source of phosphorus: detergents , industrial fertilizers and manure
Eutrophication
- Eutrophication is the ecosystem’s response to the addition of artificial or natural substances, mainly phosphates, through detergents, fertilizers, or sewage, to an aquatic system
- (Greek: eutrophia—healthy, adequate nutrition, development; German: Eutrophie)
- aka hypertrophication,.
- Negative environmental effects include hypoxia, the depletion of oxygen in the water, which may cause death to aquatic animals.
Natural eutrophication
- Very slow – occurring on geological time scale
- Climate change
- Geology
- reverse process (meiotrophication): becoming less nutrient rich with time
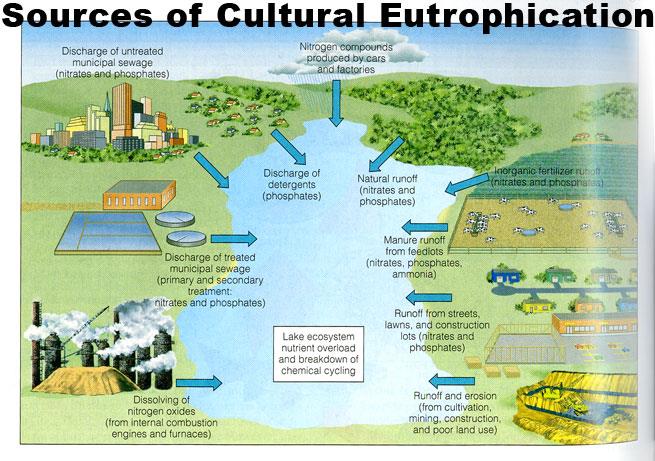
Anthropogenic/cultural eutrophication
- Runoff from agriculture and development, pollution from septic systems and sewers, sewage sludge spreading, and other human- related activities increase the flow of both inorganic nutrients and organic substances into ecosystems.
- Elevated levels of atmospheric compounds of nitrogen can increase nitrogen availability.
- Phosphorus: “point source” pollution from sewage pipes.
- Humankind has increased the rate of phosphorus cycling on Earth by four times, mainly due to agricultural fertilizer production and application.
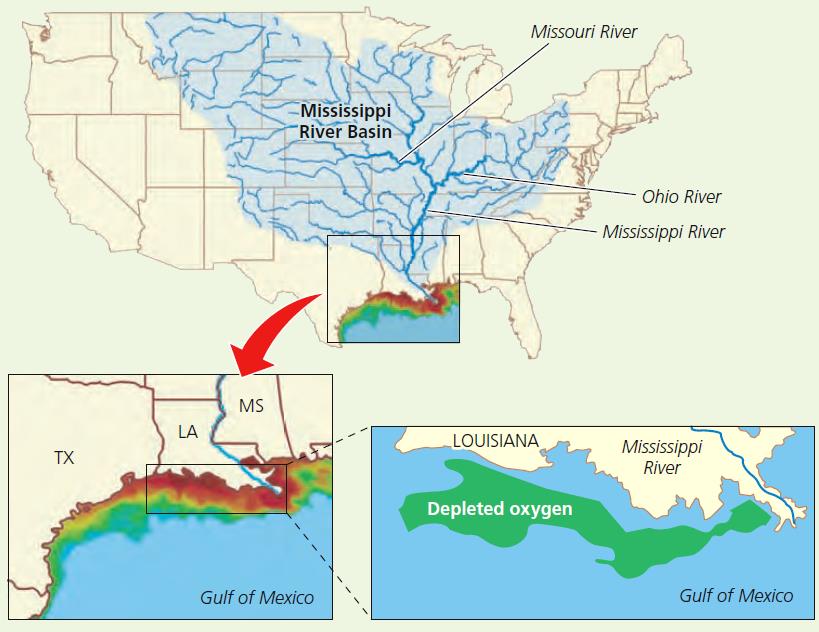
Oxygen Depletion in the Northern Gulf of Mexico
- The world’s third largest oxygen depleted zone (after those in the Baltic Sea and the northwestern
Black Sea) forms every spring and summer in a narrow stretch of the northern Gulf of Mexico off the mouth of the Mississippi River This area includes the coastal waters of the U S states of Mississippi, Louisiana, and Texas - The low oxygen levels suffocate fish, crabs, and shrimp that cannot move to less polluted areas
Salts
- Water naturally accumulates dissolved solids or salts as it passes through soils and rocks on its way to the sea.
- Most common salt cations in water are: sodium, magnesium, calcium and potassium
- Most common salt anions in water are: chloride, sulfate and bicarbonate
- Common measure of water salinity: total dissolved solids (TDS)
- Fresh water TDS limits are usually around 1,500 mg/L
- Brackish water may have TDS greater than 5,000 mg/L
- Seawater contains TDS 30,000 to 40,000 mg/L
- Drinking water has maximum TDS of 500 mg/L
- At concentrations above 2,100 mg/L water is unsuitable for irrigation
Soil Salinity
- Replacing natural vegetation with shallow rooted crops rising groundwater levels including dissolved salts
- Salt transferred into crops root zones and wetlands, streams and rivers
Salinity in Australia
- Soil salinity is a major environmental issue in Australia.
- It is a problem in most states, but especially in the south west of Western Australia.
- Some of the salts originate from marine sediments, but most have been deposited in rainfall over thousands of years.
Thermal Pollution
- Large electric power plants require enormous quantities of cooling water.
- A typical nuclear power plant can heat 150,000 m 3 hr of cooling water by about 10o C as it passes through plant condenser.
- Excessive amount of heat in the water can have adverse effects on aquatic life.
- However, within limits, thermal addition can promote fish growth.
- DO levels in water are affected by temperature changes in two main ways:
- Metabolic rates of aquatic species generally tend to increase by a factor of 2 for each rise in 10 o C temperature. This causes increased DO demand.
- Amount of DO that the water can hold at higher temperature is low.
Heavy metals
- Heavy metals are often referred to as metals with specific gravity greater than 4 or 5.
- In terms of environmental impact, the most common heavy metals are: Hg, Pb , Cd and As
- Vaporized mercury is more dangerous than liquid mercury.
- When blood containing mercury reaches the brain, it can transfer this toxic metal to the brain where it causes serious neurological damage.
- Metals are totally non degradable and therefore virtually indestructible in nature.
Pesticides
- Pesticides are a range of chemicals that kill organisms undesirable to
- They include: insecticides, herbicides, rodenticides and
- There are three main groups of synthetic organic insecticides:
- Organochlorines
- Most common organochlorine is DDT which has been widely credited with saving millions of lives (by eliminating mosquitoes, fleas and ticks )
- These are very persistent and last in the environment for a long time
- They accumulate within the food chain and become concentrated in fatty tissues of higher level organisms in the food chain
- Organophosphates
- Malathion, diazinon
- Short-lived in environment, do not bioaccumulate
- Much more toxic to humans than organochlorines that they have replaced
- Carbamates
- They are short-lived in environment
- Do not bioaccumulate
- Toxic to humans in higher exposure scenarios
Volatile organic chemicals (VOCs)
- Most commonly found groundwater contaminant
- Most important source: industrial processes
- Known to be carcinogenic
- Their volatility means they are not often found in concentrations above a few µg/L in surface water but in groundwater their concentration can be hundreds or thousands of times
- Five VOCs are especially toxic:
- Vinyl chloride: production of PVC resins
- Tetrachlorothylene: manufacture of CFCs
- Trichlorothylene: used as a cleaning agents for electronics, engines
- 1,2-dichloroethane: metal degreaser
- Carbon tetrachloride: household cleaning agent
Emerging contaminants
- Endocrine disrupting chemicals interfere with hormonal control of development in humans and wildlife
- Act like natural hormones
- Block/counteract functions of natural hormones
- Increase/decrease production of natural hormones
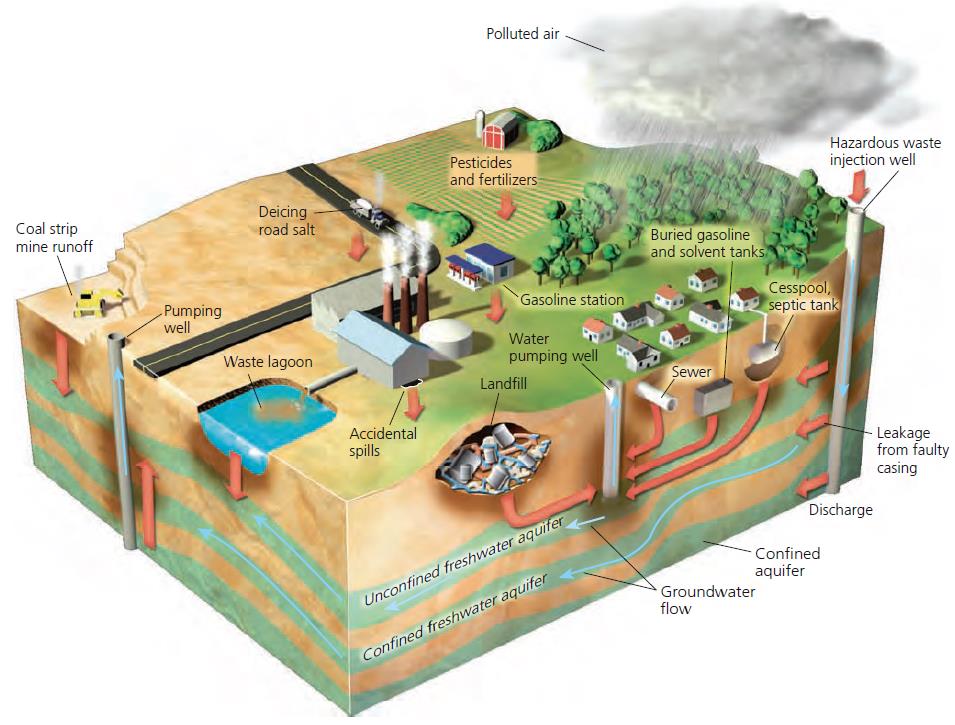
Principal sources of groundwater contamination
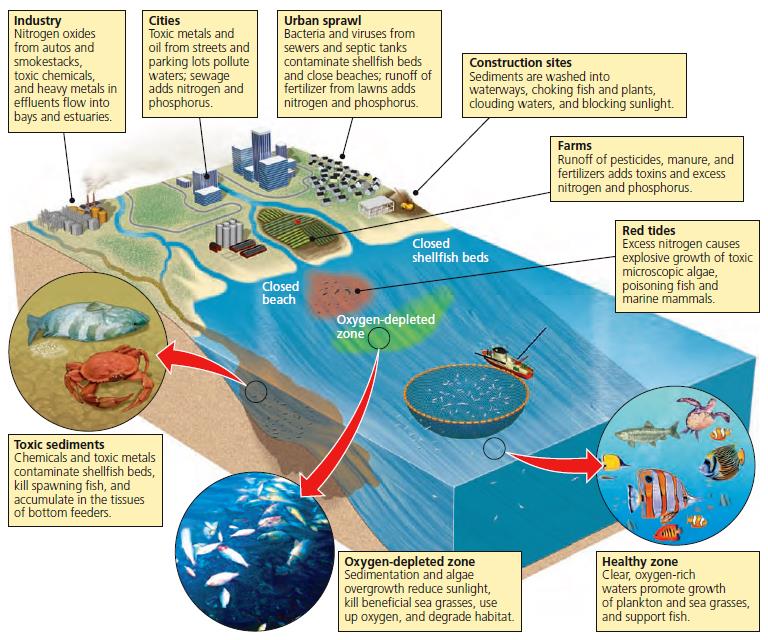
Coastal pollution
Residential areas, factories, and farms all contribute to the pollution of coastal waters and bays According to the U N Environment Programme ill health and premature death from coastal water pollution costs the world more than 30 000 a minute
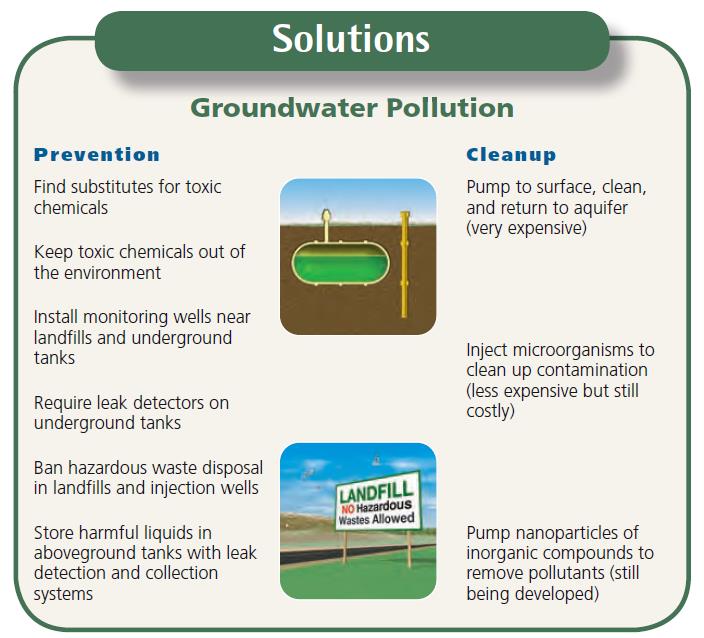
Solutions to water pollution
Controls
- Source reduction is often the cheapest and best way to reduce pollution
- Controlling nonpoint sources requires land management
- WWTP and WTP
- Ponds, lagoons, wetlands
- Water remediation may involve containment, extraction, or phytoremediation
- Water Legislation