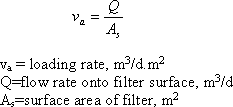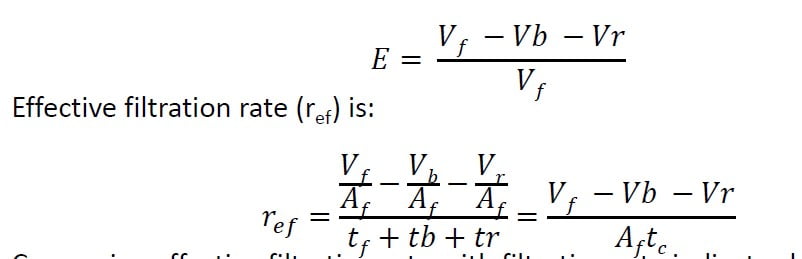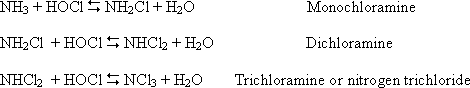Table of Contents
Water Treatment Complete Process
Since the early 1900’s sanitary engineers have been working in the United States to reduce waterborne diseases.
- In 1906 sand filters were put into use. Number of typhoid cases decreases drastically from 600 per 100,000 to about 100 per 100,000 population.
- Disinfection of the water by the addition of chlorine decreased the number of cases of typhoid further.
- A still greater decrease was accomplished after 1920 by careful control infected persons who had become carriers.
- Since 1952, death via typhoid being less than one per 1000,000 population.
From an Environmental engineering point of view, substances in water can exist in one of the three classifications:
Dissolved: A substance which is truly in solution (homogeneously dispersed in liquid). Removal of substances is not possible without phase change such as distillation, precipitation, adsorption and extraction.
Suspended: In environmental engineering, suspended solids are defined as those solids that can be filtered by a glass fibre filter disc. They can be removed by physical methods like sedimentation, filtration and centrifugation.
Colloidal: Particles in the size range between dissolved substances and suspended particles. They are in solid state and can be removed by physical means e.g. very high-force centrifugation or filtration through membranes with very small pore spaces.
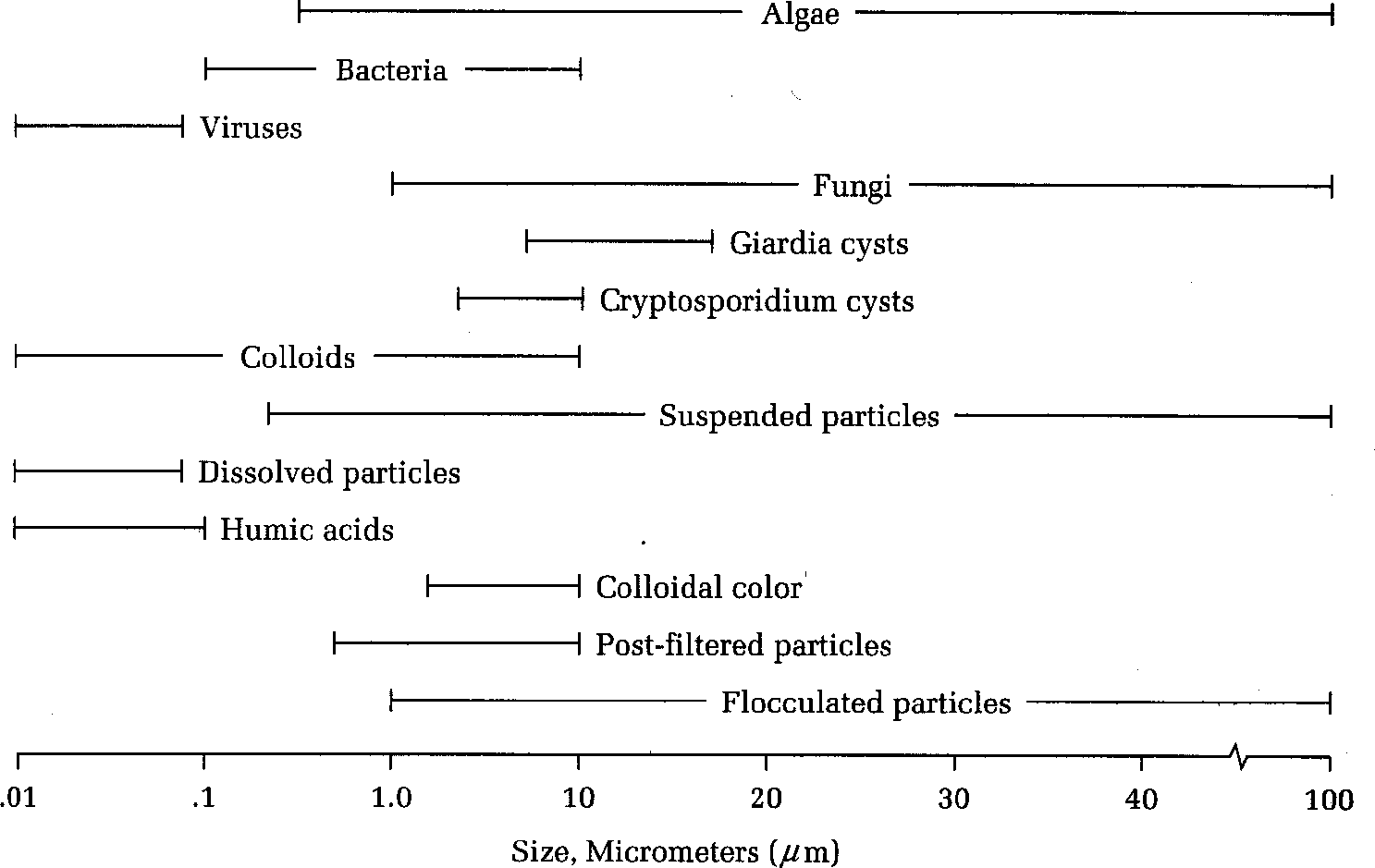
Sizes of particles in water
Quality of Water
Agents that alter the quality of water as it moves over or below the surface of the earth may be classified under four major headings:
Physical: Physical characteristics relate to quality of water for domestic use and are usually associated with appearance e.g. color, taste, odour or temperature.
Chemical: Differences in water evidenced by their observed reactions e.g. performance of hard and soft water in laundering.
Biological: Biological agents important from public health perspective and may also modify physical and chemical characteristics of water.
Radiological: Considered only when water has come in contact with radioactive substances.
Treatment Systems
Treatment systems can be into three categories:
Disinfection Plant: Plants employing simple chlorination. Water is usually of high
quality and chlorination is done to ensure safe bacteria level in water.
Filtration Plant (surface water treatment): Rapid mixing, flocculation, sedimentation, filtration and disinfection employed to remove color, turbidity, taste and odour, and bacteria. During rapid mix, chemicals are added and rapidly dispersed to join desired impurities and form precipitates (flocs).
Softening Plant (ground water treatment): During rapid mixing, chemical doses (much higher) are added to react with the hardness and precipitate it. After sufficient reaction time, precipitate will collide and grow in size in reaction basin. The other unit operations are same for both except recarbonation.
Ground water and Surface water Composition
Groundwater (deep/shallow wells)
- constant composition
- high mineral content
- low turbidity and color
- no D.O. & bacteriologically safe
- high hardness
- Fe, Mn, H2S
Surface water (Rivers, lakes,Reservoirs)
- variable composition
- low mineral content & hardness
- high turbidity
- color
- D.O. & microorganisms present
- taste and odor
- Possible chemical toxicity
Coagulation
Coagulation is the process by which particles become destabilized and begin to clump together. The object of coagulation (and subsequently flocculation) is to turn the small particles of color, turbidity and bacteria into larger flocs, either as precipitates or suspended particles.
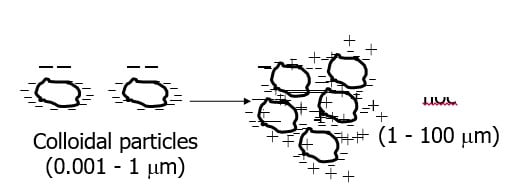
Colloids
Small particles (0.001 to 1 micro m)
Usually negatively charged
Particles repel so suspension is considered stable
Colloidal particles (0.001 – 1 micro m)
Colloid Stability
The particles in the colloid range are too small to settle in a reasonable time period and too small to be trapped in the pores of a filter. Most colloids are stable because they possess a negative charge that repels other colloids particles before they collide with one another.
Colloid Destabilization
Since colloids are stable because of their surface charge, in order to destabilize the particles, we must neutralize this charge. Such neutralization can take place by addition of an ion of opposite charge to the colloid. Since most colloids found in water are negatively charged, the addition of a divalent or trivalent should reduce the charge.
Collision efficiency factor α measures the degree of destabilization of colloids. If every collision results in aggregation then α= 1.0.
Goal
To alter the surface charge of the particles that contribute to color and turbidity so that the particles adhere to one another and are capable of settling by gravity.
How is this goal achieved?
By adding a coagulant. The key properties of a coagulant are:
- Trivalent cation
- Non toxic & inexpensive
- Insoluble in neutral pH range
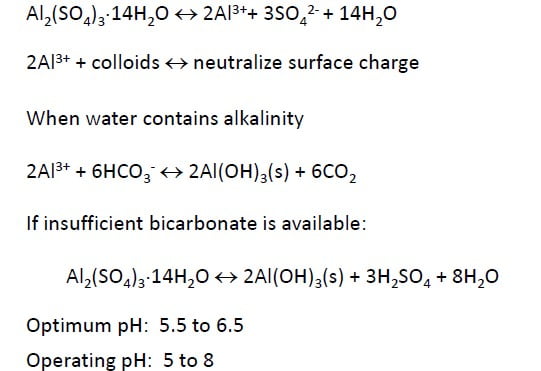
Usually Alum (Al2(SO4)3.14H2O) is used. Sometimes Ferrous sulfate (FeSO4) , Ferric Chloride (FeCl3) and polyelectrolytes are also used.
Step in Water Treatment
1) Coagulation process
Factors Which Affect the Coagulation
The factors which affect coagulation are :
- Kind of coagulant
- Quantity of coagulant
- Amount, character of colour and turbidity of water
- pH value of water
- Time of mixing and flocculation
- Temperature
- Violence of agitation
Function of Coagulation
These are to remove :
-
- Turbidity
- Organic and inorganic matter
- Colour
- Harmful and other pathogenic bacteria
- Algae, planktons and other organisms
- Taste and odour producing substances
How does alum work?
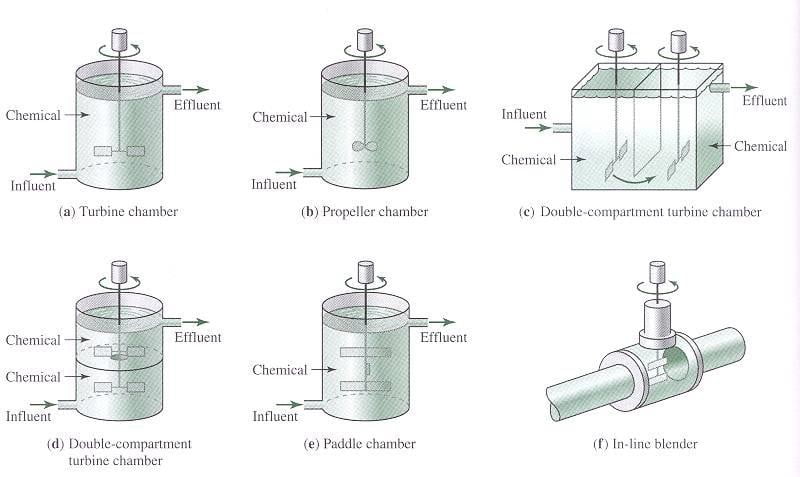
2) Rapid Mixing
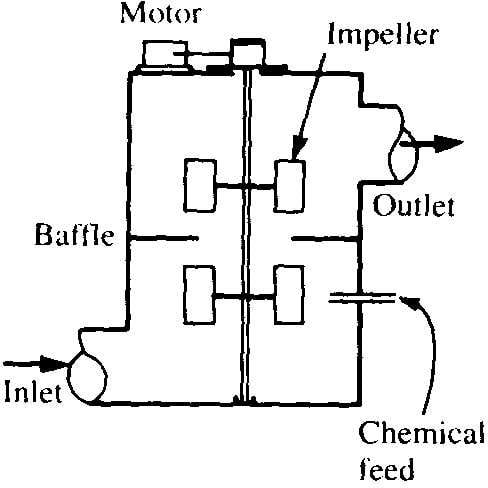
- Rapid mixing involves dispersing a coagulant through the raw water rapidly and uniformly.
- Mixing achieved mechanically using vertical-shaft impeller in tank with baffles
- Chemicals are different for surface water (coagulation) or ground water (softening)
- Detention time (td):
- 10 – 30 sec for coagulation
- The time needed to achieve efficient coagulation varies
- Depending on the coagulation mechanism involved.
In the treatment of water and wastewater the degree of mixing is measured by the velocity gradient, G.
The total number of particle collisions is proportional to G.t0, where t0 is the detention time in the basin. In ideal reactors the time in the reactor (detention time or retention time) is defined as :
Typical Mixing Configurations
3) Flocculation
During coagulation the chemical reactions that take place in rapid mixing form precipitates, such as aluminum hydroxide or iron hydroxide. The precipitates formed in these processes must be brought into contact with one another so that they can agglomerate and form larger particles, called flocs. This contacting process is called flocculation and is accomplished by slow, gentle mixing.
While rapid mix is the most important physical factor affecting coagulant efficiency, flocculation is the most important factor affecting particle removal efficiency. The objective of flocculation is to bring the particle into contact so that they will collide, stick together, and grow to size that will readily settle. Enough mixing must be provided to bring the floc into contact and to keep the floc from settling in the flocculation basin.
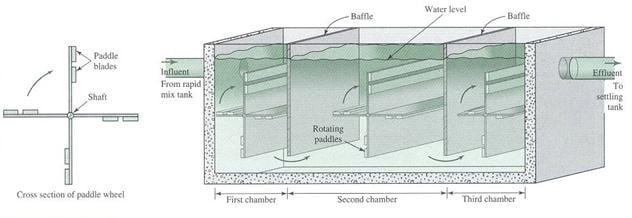
Flocculator
Flocculation is normally accompolished with an axial – flow impeller), a paddle flocculator or a baffled chamber. The baffle-mixing basin is called also “up and down baffle mixing basin”.
Paddle units rotate slowly, usually <1 rpm Velocity of water: 0.5 – 1.5 ft/sec Detention time of at least 20 min
Paddle Flocculator
Baffled Chamber Flocculator
4) Sedimentation
Particles that will settle within a reasonable period of time can be removed in a sedimentation basin (also called clarifier).
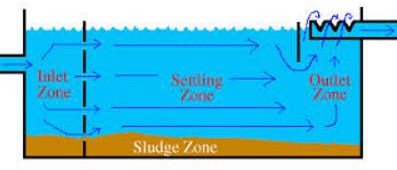
Sedimentation basins are usually rectangular or circular with either a radial or upward water flow pattern. Regardless of the type of basin, the design can be divided into four zones; inlet, settling, outlet, and sludge storage.
Inlet zone: The purpose of the inlet zone is to evenly distribute the flow and suspended particles across the cross section of the settling zone. The inlet zone consists of a series of inlet pipes and baffles extending about 1 m into the tank.
Settling zone: Sedimentation efficiency does not depend on the tank depth. The forward velocity should be low enough so that the settled material does not re-suspend from the tank floor. Water velocity lowers to design velocity of settling upon entering the zone.
Outlet zone: The outlet zone is designed so as to remove the settled water from the basin without carrying away any of the floc particles.
Sludge storage: The configuration and depth of the sludge storage zone depends upon the method of cleaning, the frequency of cleaning, and the quality of sludge estimated to be produced.
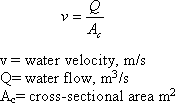
To remove the water from the basin quickly, it is desirable to direct the water into a pipe or small channel for easy transport, which will produce a significantly higher velocity. Rather than put a pipe at the end of the sedimentation basin, it is desirable to first put a series of troughs, called weirs, which provide a large area for the water to flow through and minimize the velocity in the sedimentation tank near the outlet zone. The weirs then feed into a central channel or pipe for transport of the settled water.
Sedimentation Concepts
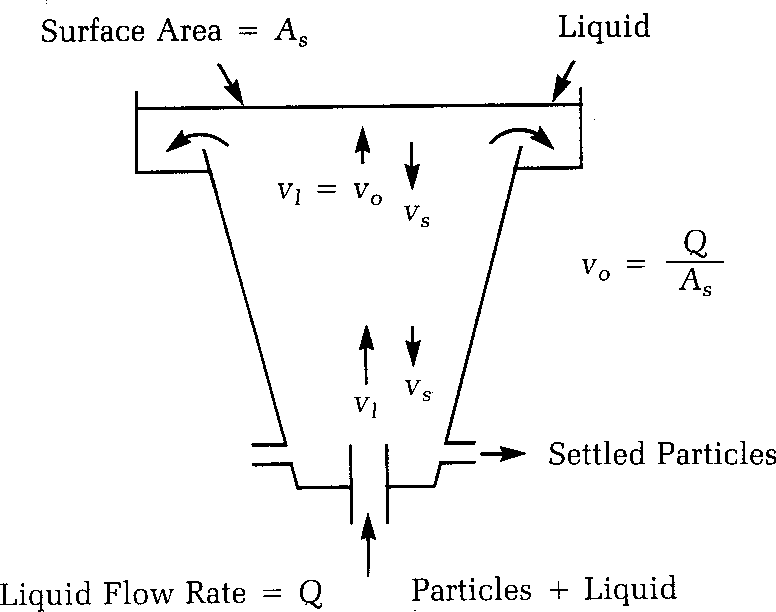
There are two important terms to understand in sedimentation zone design. The first is the particle (floc) settling velocity, vs. The second is the velocity at which the tank is designed to operate, called the overflow rate, v0. The easiest way to understand these two concepts is to view a upward-flow sedimentation tank.
VO = Q /AC
where
V0 = overflow rate (m/s)
Q = water flow (m3/s)
Ac = surface area (m2)
In this design, the particles fall downward and the water rises vertically. The rate at which the particle is settling downward is the particle-settling velocity, and the velocity of the liquid rising is the overflow rate.
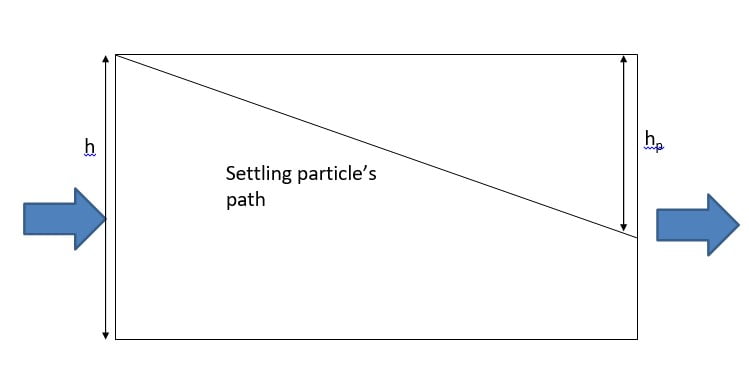
Trajectory of a particle settling as it is carried from left to right by flow of
water through a sedimentation basin
Settling properties of Particle
Particle settling velocity is different for different particles. Settling properties of particles are often characterized into four types:
Type I: settling applies to particles that settle discretely with constant velocity. They don’t flocculate or stick e.g. sand and grit material
Type II: In type II particles flocculate during settling. As flocculating, size is continuously changing, therefore, velocity also changes. It generally increases
e.g. alum or iron coagulation
Type III: In type III particles concentration increases with depth, zone settling occurs. Particle concentration are higher than 1000 mg/L such that particles tend to settle as a mass thus forming a distinct clear zone and sludge zone.
Example, lime softening sedimentation and activated sludge sedimentation
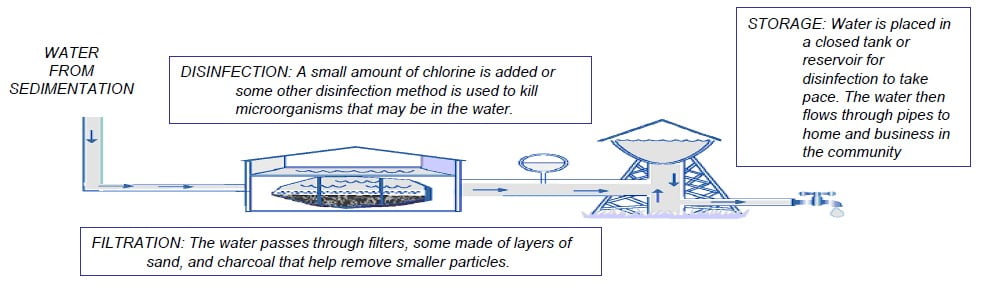
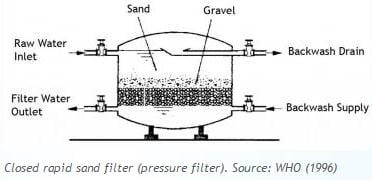
5) Filtration
Water flows through a filter designed to remove particles in the water. The filters are made of layers of sand, gravel and in some cases crushed anthracite. Filtration collects the suspended impurities in water and enhances the effectiveness of disinfection. Filtration provides the additional opportunity for separation of small flocs or particles. The filters are routinely cleaned by backwashing.
Classification of Filters
There are several methods of classifying filters. One way is to classify them according to the type of medium used such as:
- Single media: sand or coal
- Dual media (coal plus sand)
- Mixed media (anthracite, coal, sand and garnet)
Another common way to classify the filters is by allowable loading rate. Loading rate is the flow rate of water applied per unit area of the filter. It is the velocity of the water approaching the face of the filter :
Effective filtration rate
Some clean water in every cycle must be used for backwash and to rinse the filter after backwash.
Therefore, less than 100% water is usable.
Filter efficiency or production efficiency (E) is used to measure filter performance.
Comparing effective filtration rate with filtration rate indicates how much
overall throughput rate has decreased due to backwash.
Based on loading rate, the filters are described as being slow sand filters, rapid sand filters, or high-rate sand filters.
Slow sand filters: Water is applied to sand at a loading rate of 2.9 to 7.6 m3/day.m2. Particles begin to collect in the top 75 mm and clog the pore spaces. As the pores become clogged, water will no longer pass through the sand. At this point the top layer of sand is scraped off, cleaned and replaced. Slow sand filters require large areas of land and are operator intensive.
Rapid sand filters: Water is applied at a loading rate greater than or equal to 120 m3/day.m2. These filters have graded (layered) sand within the bed. The sand grain size distribution is selected to optimize the passage of water while minimizing the passage of particulate matter. Rapid sand filters are cleaned through backwashing. They are most commonly used filters.
In the filter upto 99.5 % of the Suspended Solids can be removed from the water including minerals, flocs and microorganisms.
The settled water has turbidity ranging from 1 to 10 NTU which is reduced to
0.3 NTU in filtration. Filtration collects the suspended impurities in water and enhances the effectiveness of disinfection. It also provides the additional opportunity for separation of small flocs or particles.
6) Disinfection
Disinfection is the killing of disease causing micro-organisms. In the process coliform bacteria will be killed too and the total bacterial count (TBC) will be reduced. Disinfection is used in water treatment to reduce pathogens (disease producing microorganisms) to an acceptable level.
Disinfection Kinetics
Under ideal conditions, when an exposed microorganism contains a single site vulnerable to a single unit of disinfectant, the rate of die-off follows Chick’s law, which states that the number of organisms destroyed in a unit time is proportional to the number of organisms remaining :
. . . . (first order reaction)
7) Chlorination
Chlorine is the most common disinfecting chemical used. Chlorine is used in the form of compressed gas under pressure or sodium hypochlorite (NaOCl), or as calcium hypochlorine [Ca(OCl)2]. When chlorine is added to water, a mixture of hypochorous acid (HOCl) and hydrochloric acid (HCl) is formed :
Cl2 (g) + H2O = HOCl + H+ + Cl-
pH dependent and essentially completes within a few milliseconds
HOCl = H+ + OCl-
HOCl is about 80 – 100 times more effective than is OCl- for E. Coli Chlorine in the form of [HOCl] and [OCl-] is called free available chlorine
Hypochlorite reactions
Chlorine is a strong oxidizing agent and reacts with organics and inorganics in water to form chloramines (NH2Cl (mono) and NHCl2 (di chloramine)). Di- and tri- chloramines are undesirable for taste and odour. Combined chlorine’s use has been encouraged as free chlorine contribute to the production of THMs (CHF3, CHCl3, CHBrCL2).
Chlorine destroys the extracellular system of cells. The dosages are low as 0.25-0.5 mg/l.
Low temperature and high pH adversely affect the efficiency of chlorine.